Lewis Structure For Co2 Lone Pairs
Then weve got one assigned to the formal negative charge. Divide that by two.
The Lewis Structure For Carbon Monoxide Is C O Chegg Com
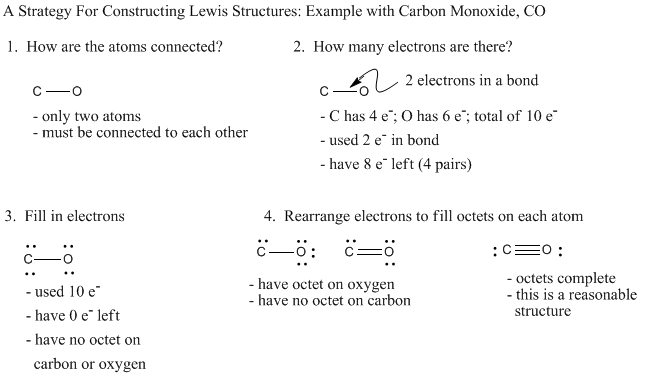
In a Lewis structure some of the electrons are bonding electrons and others are non-bonding electrons known as lone pairs Here is a simple step by step process using CO2 as an example.
Lewis structure for co2 lone pairs. Include all lone pairs. In Lewis Dot structure for CO2there are four lone pairsEach oxygen atom has two lone pairs around itThe carbon atom doesnt have any lone pair. Include lone pairs on all atoms where appropriate.
Co2 Molecule Below Is The Lewis Structure Of The V alence S hell E lectron P air R epulsion Theory Unit 6 PowerPoint Presentation ID2168454. But each oxygen in the CO2 lewis dot structure has two lone pairs. Which Lewis structure is correct for CO2.
A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Shape of CO2 is linear. Carbon is the least electronegative that means it stays at the center.
No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Drawing correct lewis structure is important to draw resonance structures of CO 3 2-correctly. Once we have a Lewis Structure f.
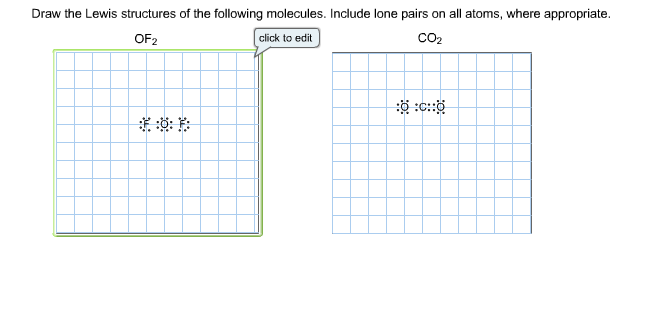
Each oxygen makes 1 sigma bond and also needs 2 orbitals. So total valence electrons are 16. HF Н0 Select Draw Rings More Erase Select Draw Rings More 2 3 HNO Select Draw Rings More Erase 2 o Draw the Lewis structure for each molecule.
Now structure wise that will look are the following where each of the oxygens will have three low pairs to have feeling six lone pairs. Total number of electrons of the valance shells of CO 3 2. There are two double bonds around carbon atom in the CO2.
Put lone pairs on atoms. Check the stability and minimize charges on atoms by converting lone pairs to bonds. We have 20 electrons.
Draw the Lewis structure of each molecule. Co2 Lewis Structure Lone Pairs The Lewis Dot Structure for CO2 Solved. Now that we formed a double bond between one of the carbons and the oxygen carbon still does not have a full octet.
This atom will be 2sp hybridized with remaining 2px and 2py atomic orbitals. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase. So this is the correct Lewis structure for CO2.
CO2 Lewis structure So CO2 4 62 16. So lets form a double bond between the other oxygen and the carbon. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
The carbon atom has no lone pairs. In Lewis Dot structure for CO2the carbon atom follows the octet rule and two oxygen atoms also follow the octet rule. The carbon-oxygen bonds are double bonds.
From the Lewis structure we can see that the carbon in CO2 must make 2 sigma bonds and it has no lone pairs. Weve got 10 potential electron pair bonding or lone pairs. To determine the number of lone pairs and bonding pairs of electrons for CO2 we first need to draw as valid Lewis Structure.
Draw the Lewis structures of the given molecules. Steps of drawing lewis structure of CO 2 Determine total number of electrons of the valance shells of carbon and oxygen atoms Total electrons pairs existing as lone pairs and bonds Determine center atom and drawing the sketch Mark lone pairs on atoms Mark charges on atoms if there are charges on. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase 0 F С.
0 5 Complete these structures by adding electrons in the form of dots as needed. Now each atom in our structure has a full octet. CO2 lewis dot structure contains two oxygen atoms and one carbon atom connected with the double bond whereas carbon is the central atom and no lone pair is present on it.
Carbon Dioxide is a Linear molecule with AX2 geometry a linear shape and a 180 degree bond angleCheck me out. A Lewis structure is a way to draw out electrons and bonding by using dots. Now we have three bonding pass because we have three act IMS that will bond to the central carpenter Got to Oxygens and one hydrogen the meaning that we have six lone person.
In the CO2 molecule each oxygen atom has two lone pairs of electrons. Carbon dioxide CO2 lewis structure has two oxygen atoms and one carbon atom.

Lone Pairs Bonding Pairs And In The Lewis Structure Chegg Com

How To Draw Co2 Lewis Structure Science Education And Tutorials
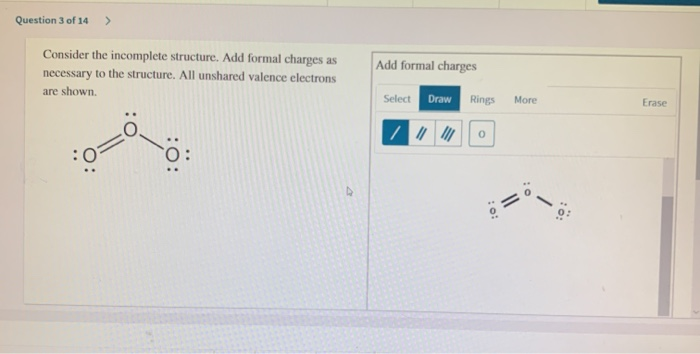
Estion 1 Of 14 Draw The Lewis Structures Of The Chegg Com
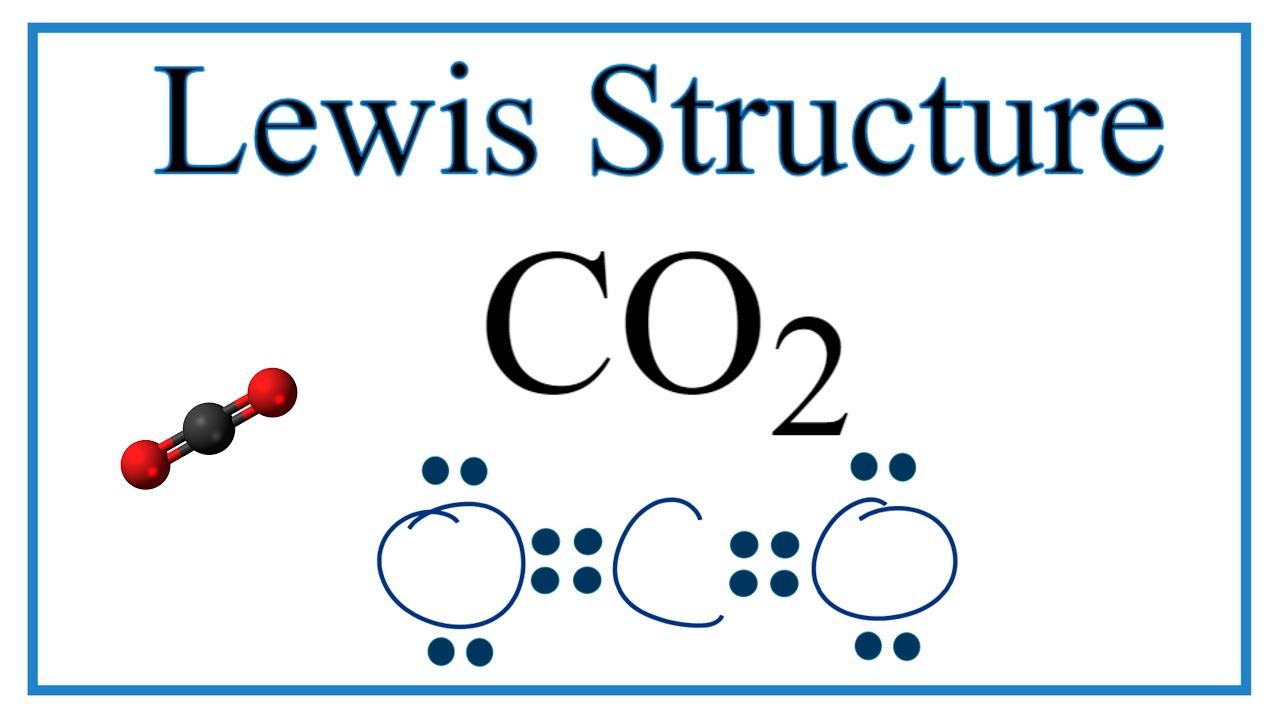
Number Of Lone Pairs And Bonding Pairs For Co2 Carbon Dioxide Youtube

Draw The Lewis Structures Of The Following Molecules Chegg Com
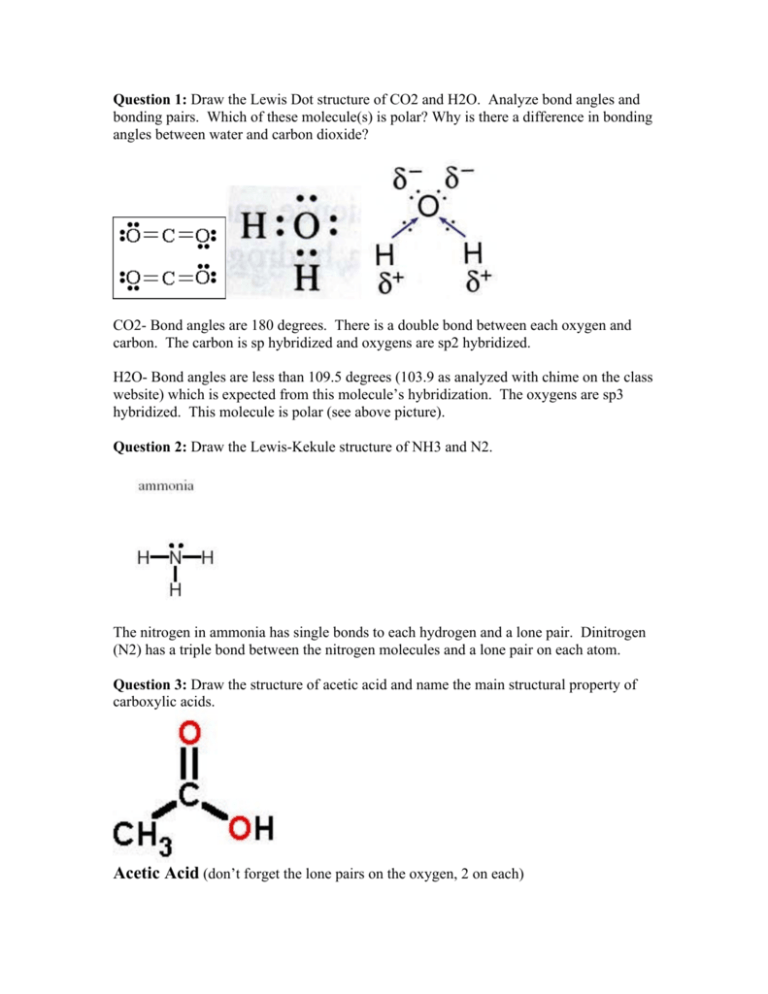
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Lone Pair On Carbon In Co2 Chemistry Stack Exchange
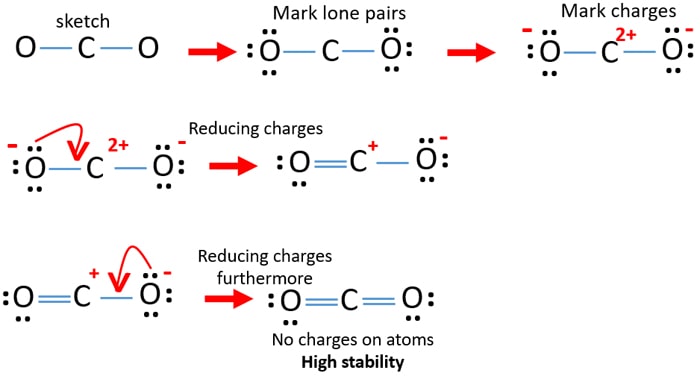
Co2 Carbon Dioxide Lewis Structure And Shape
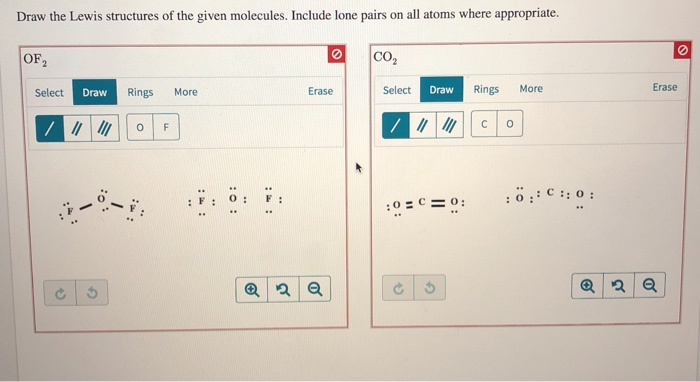
Draw The Lewis Structures Of The Given Molecules Chegg Com
How Many Pairs Of Electrons Should Be Drawn In The Lewis Structure For Carbon Dioxide Co2 Quora

Number Of Lone Pairs And Bonding Pairs For Co2 Carbon Dioxide Youtube

Unit 5b Covalent Bonding Ppt Video Online Download

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization
Co2 Lewis Structure Molecular Geometry And Hybridization

Lewis Structures In Covalent Bonds Valence Electrons Are Distributed As Shared Or Bond Pairs And Unshared Or Lone Pairs H Cl Shared Or Bond Pair Ppt Video Online Download
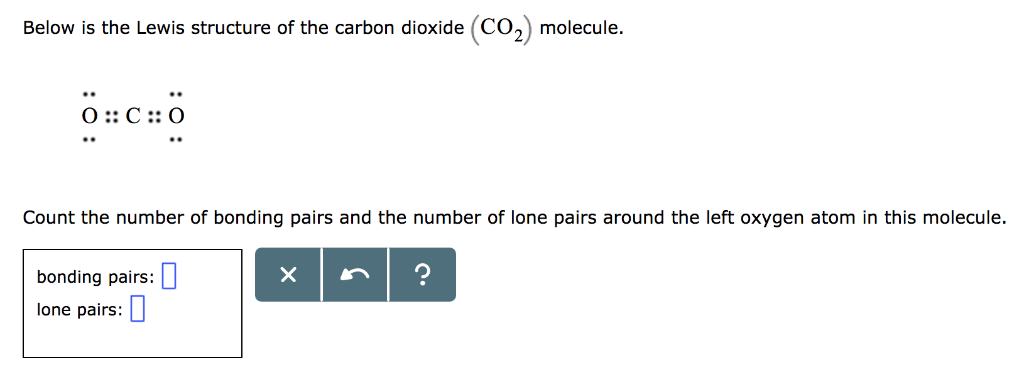
Co2 Molecule Below Is The Lewis Structure Of The Chegg Com
How Many Pairs Of Electrons Should Be Drawn In The Lewis Structure For Carbon Dioxide Co2 Quora

Carbon Dioxide Co 2 The New Lewis Structure Specifies That The Two Download Scientific Diagram