Phosgene Gas Lewis Structure
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. Include all three resonance forms by alternating the double bond among the three terminal atoms.

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemical Weapon During World War I And It Is Brainly Com
In a study of 63 industrial effluents that discharge into surface waters chlorobutane was found in one effluent at a concentration of.

Phosgene gas lewis structure. Phosgene is an extremely toxic gas used as a chemical weapon during World War I. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. There are no remaining electrons hence no need to place them on central atom.
Phosgene cl2co is a poisonous gas that was used as a chemical weapon during world war i and it is a potential agent for chemical terrorism. Phosgene is a valued industrial building block especially for the production of precursors of polyurethanes and polycarbonate plastics. Write the Lewis structures for carbon tetrachloride and phosgene.
Phosgene is the organic chemical compound with the formula COCl 2. Therefore P 6n 2 V 6 4 2 24 2 There are 2 π electrons pi electrons in COCl2 and so 1 double bond must be added to the structure of Step 1. Identification of Organic Compound in Industrial Effluent Discharges USEPA-6004-79-016 NTIS-PB-294794 1979 Hazardous Substances Data Bank HSDB.
One double bond must therefore be placed to the structure in Step 1. The number of valance electrons is Step. With cooling and pressure phosgene gas can be converted into a liquid so that it can be shipped and stored.
The CO distance is 118 Å the CCl distance is 174 Å and the ClCCl angle is 1118. Phosgene is a major industrial chemical used to make plastics and pesticides. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 9892 grammol.
It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. Draw the Lewis structure of phosgene. Step 3 4.
2 draw a line between each pair of connected atoms to represent the electrons in a covalent bond. It is one of the simplest acyl chlorides being formally derived from carbonic acid. A dash or line is sometimes used to indicate a shared pair of electrons.
Include all three resonance forms by alternating the double bond among the three terminal atoms. PROBLEM PageIndex7 The arrangement of atoms in several biologically important molecules is given here. Draw molecules by placing atoms on the grid and connecting them with bonds.
Draw the lewis structure of phosgene. In low concentrations its odor resembles that of freshly cut hay or grass. A dash or line is sometimes used to indicate a shared pair of electrons.
A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure PhosgeneFor the COCl2 structure use the periodic table to find the total number of. Phosgene is a planar molecule as predicted by VSEPR theory. It is a potential agent for chemical terrorism today.
When liquid phosgene is released it quickly turns into a gas that stays close to. Add lone pairs so that each atom connected to the central atom gets an octet. It is also used in the manufacture of dyestuffs some insecticides and pharmaceuticals and in metallurgy.
COCl2 Lewis Structure Molecular Geometry Hybridization and Polarity. In the past phosgene was used as a chemical. It is non-flammable in nature and bears a suffocating odor.
Where V 4 6 7 7 24. COCl2 is a chemical compound known by the name phosgene. It is now an important industrial starting material for the synthesis of Lexan a lightweight transparent material used in bike helmets goggles and catchers masks.
Phosgene is used for the synthesis of isocyanate -based polymers carbonic acid esters and acid chlorides. Structure and basic properties. Phosgene Cl2CO is a poisonous gas that was used as a chemical weapon during World War I.
Complete the Lewis structures of these molecules by adding. At room temperature 70F phosgene is a poisonous gas. Phosgene Cl2CO C l 2 C O is a poisonous gas used as a chemical weapon during World War I.
1 Perry DL et al. Include all nonbonding electrons. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms.
Draw a valid Lewis structure for phosgene CCl 2 O which contains a central carbon atom. It is a potential agent for chemical terrorism today. It is a colorless gas.
Draw the Lewis structure of phosgene. A dash or line is sometimes used to indicate a shared pair of electrons.

Describe The Hybridization Of The Carbon Atom In The Poisonous Gas Phosgene Cl2co Study Com
Solved Draw A Valid Lewis Structure For Phosgene Ccl 2 O Which Contains A Central Carbon Atom Phosgene Is An Extremely Toxic Gas Used As A Chemi Course Hero

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
4 2 Lewis Structures Problems Chemistry Libretexts

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
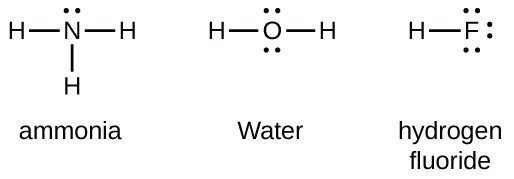
Lewis Symbols And Structures Chemistry For Majors

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Day03 3 Resonance Structure Of Phosgene Cocl2 Youtube

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemical Weapon During World War I And It Is Brainly Com
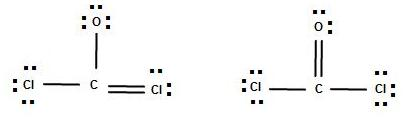
The Follosing Are Two Lewis Structures That Can Be Chegg Com

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube
How To Calculate The Formal Charge Of Cocl2
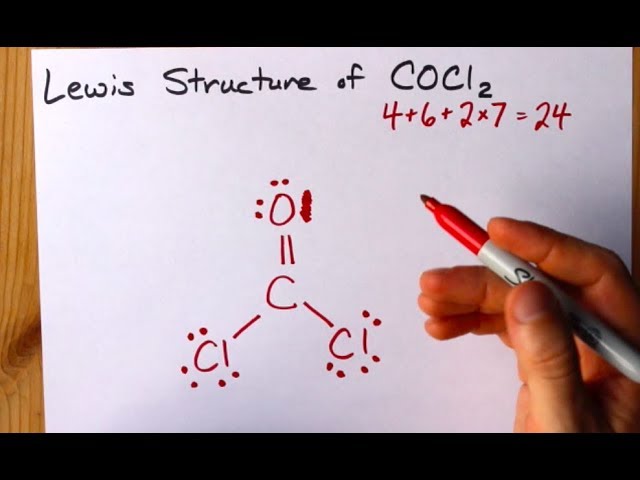
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemi Clutch Prep

Day03 3 Resonance Structure Of Phosgene Cocl2 Youtube

6 3 Molecular Shape Introductory Chemistry

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemi Clutch Prep
4 2 Lewis Structures Problems Chemistry Libretexts