Brf3 Lewis Structure Formal Charge
A the electron pairs on Br b molecular geometry c formal charge on Br d the polarity of the molecule polar or non-polar 2. 1 two lone pairs on Br seesaw without lone pairs geometry formal charge on Br is zero The structure of BrF3 is not symmetric so it is polar.
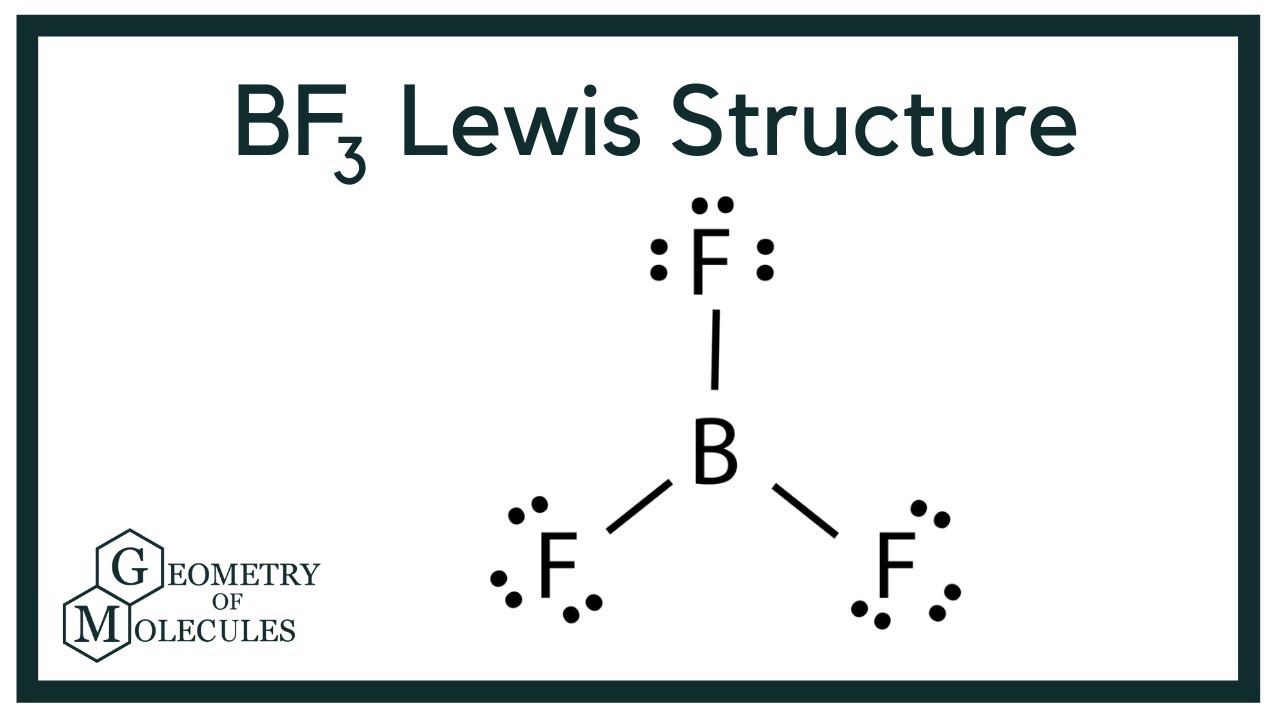
Bf3 Lewis Structure Boron Trifluoride Youtube
C valence Shell electrons of Atom - Number of bonds associated with the Atom - No.

Brf3 lewis structure formal charge. April 11th 2019 - This is the BrF3 Lewis structure We have a total of 28 valence electrons for the BrF3 Lewis structure Bromine is the least electronegative we ll put that at the center and then we ll put the Fluorines around the outside We ll put single bonds between the Fluorines and the Bromine That s six valence 2 4 6 valence electrons we ve used. Both need 1 extra electron to complete their octets. So all the formal charges are zero and this is the Lewis structure for BrF3 and this is Dr.
The Bromine has more than 8 but thats OK. There are a total of 28 valence electrons for the BrF 3 Lewis structure. In order to calculate the formal charges for BF3 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec.
There are a total of 28 valence electrons for the BrF 3 Lewis structure. Bromine trifluoride chemical formula is BrF3. Of course BF 3 is still a Lewis acid and in fact you can form Et2O BF 3 boron trifluoride etherate as a distillable liquid.
To know about BF3 Lewis structure we have to calculate the total number of valence electrons for the BF3 molecule. If we check the formal charges for the BF 3 Lewis structure we will find that they are zero even though B only had six valence electrons. For the BrF 3 Lewis structure youll need to put more than eight valence electrons on the Bromine atom.
What Is The Formal Charge On The Bromine Atom. In the BrF 3 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure. BF3 has a total of 24 valence electrons which we have to set around the central atom.
Very toxic by inhalation and corrosive to metals and tissue. Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the structure. In the Lewis structure for BrF 3 there are a total of 28 valence electrons.
Let us calculate for BrF3. What is the formal charge on the central Cl atom. 3 C K and Cl are isoelectronic.
Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at the center of the. Of Onshared electron by it 8 8 7 - 3 - 40 F 7 - 1 - 6 0 F 7 - 1 - 60 F 7- 1- 60 - Does not have any Resonance Structures. This problem has been solved.
Sketch the Lewis structure of the molecule BrF3 showing in detail. Both have Ne view the full answer. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets.
Draw the best Lewis structure for Cl3-. Boron is an exception and only needs 6 valence electrons in its outer shell. Bromine trifluoride appears as a colorless to yellow fuming liquid with a pungent odor.
I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 I- - I3-. What is the formal charge on the bromine atom.
Cl - Cl - Cl -. For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule. This is how we calculate the formal charge.
BrF3 Lewis Structure - How to Draw the Lewis Structure for BrF3 - YouTube. I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. In Lewis Structure formation we have to check whether all the atoms have their least possible formal charge values.
B and thanks for watching. Here in this post we described step. BX Ez A Lewis Structure - Total Valence electrons 77x3 28 i F - Bx - E.
BX3 X ClBr tend to be so. Drawing BrF3 Lewis Structure is very easy to by using the following method. Formal Charge 7- 05 2 -6 0.
Textbook Solutions Expert QA Study Pack Practice Learn. And if we calculated the formal charges wed see that the formal charges on each of the atoms in the Lewis structure for BrF3 is zero. Before completing the octets dont forget to determine how many valence electrons there in Boron Trifluoride and place them accordingly.
F Formal Charge. Formal Charge is the charge given to constituent atoms inside a chemical molecule where the bonding is shared equally among all the atoms present. Answer to Draw the Lewis structure for BrF3.
Draw The Lewis Structure For BrF3. - Formula for Calculate the formal Charge is- F. Which of the following represents the lewis structure for Ca 2.
Which of the following represents the lewis structure for Mg. BrF3 CS2 SiF4 SO3. Containers exposed to prolonged heat may violently rupture and rocket.
A video explanation of how to draw the Lewis Dot Structure for Bromine Trifluoride along with information about the compound including Formal Charges Pola. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings.
Answer In General Chemistry For Brittany Wallace 98925

Asf5 Lewis Structure How To Draw The Lewis Structure For Asf5 Youtube

How To Calculate The Formal Charges For Bf3 Boron Trifluoride Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
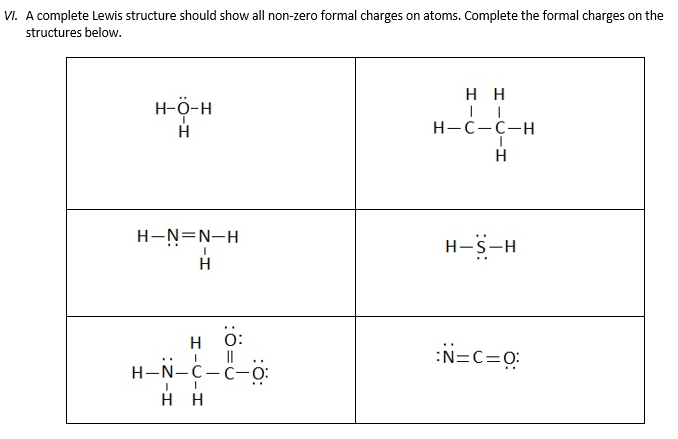
V Using The Steps For Determining Lewis Structure Chegg Com

Brf3 Lewis Structure Bromine Trifluoride Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
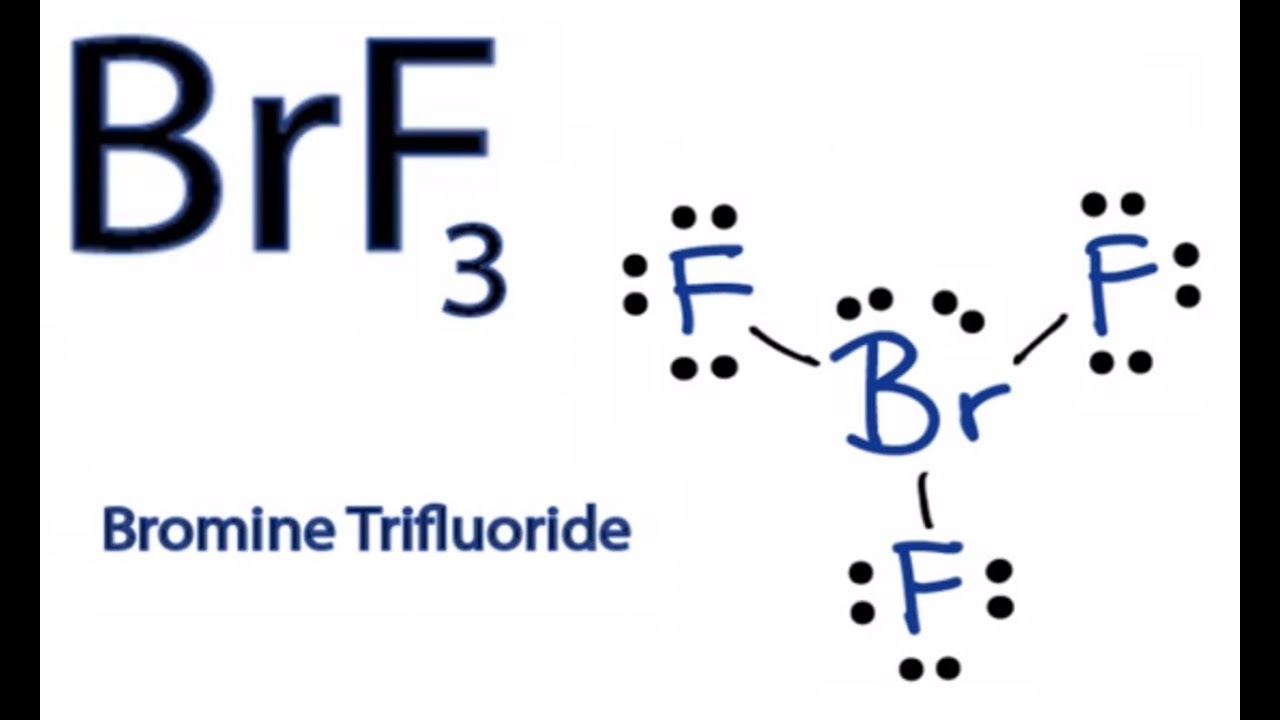
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
Answer In General Chemistry For Brittany Wallace 98925
Answer In General Chemistry For Brittany Wallace 98925
Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Bf3 Lewis Structure How To Draw The Lewis Structure For Bf3 دیدئو Dideo

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
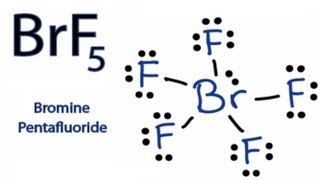
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
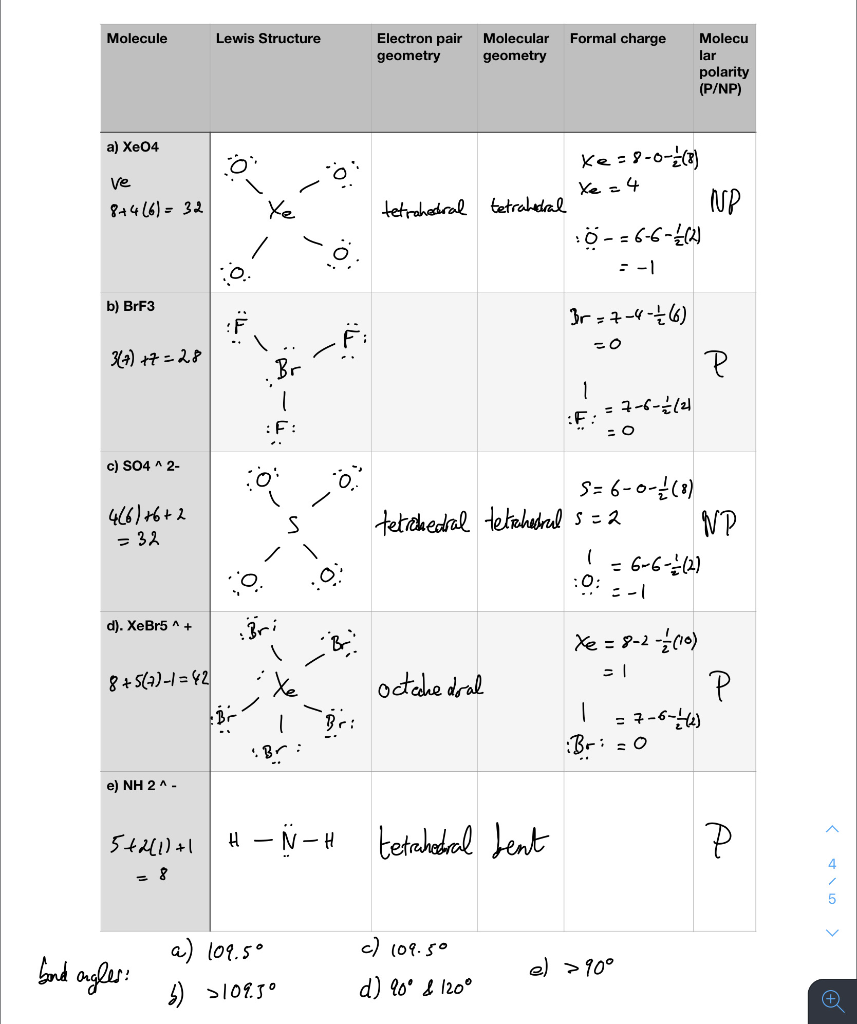
Oto Molecule Lewis Structure Formal Charge Electron Chegg Com

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
