Lewis Structure Of C2h4cl2
Expert Answer 100 1 rating Previous question Next question. Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar.
4 2 Lewis Structures Problems Chemistry Libretexts
Calculate the total number of valence electrons present.
Lewis structure of c2h4cl2. Draw Lewis structures for each molecular formulaa. By signing up youll get thousands of. Compound Total of Valence Electrons Lewis Structure Resonance Structures H2SO4 HCOOH COCl2 C2H4Cl2 Lewis Structures Names Data Sheets.
This problem has been solved. Count total valence electron in C2H4. It is used as a chemical intermediate in the production of vinyl chloride and of 111-trichloroethane.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. No lone pair is present on the central or outer atom in the lewis structure of ethene. How Many Shared Electron Pairs Are There In The Lewis Structure Of C2H4Cl2.
To do so we first need to do the following steps. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. See the Big List of Lewis Structures.
However the complete structural. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Is it polar or nonpolar.
Hydrogen has 1 valence electron but we have two Hydrogens. Plus Chlorine which is 7 and we have two Chlorines for a total of 24 valence electrons. The complete structural formula shows all of the atoms and exactly how they are connected.
Ion Total Valence Electrons Lewis Structure Resonance Structures CN- CO32- SO42- NO21- Lewis Structures Names Data Sheets. There are various types of formulas that one can write for a particular molecule. Structure properties spectra suppliers and links for.
Draw the Lewis structure for C2H4Cl2 and state its molecular geometry. The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1. According to the octet rule a molecule should have eight electrons in its outer shell to become inert or stable.
The structure of C 2 H 4 molecule is shown below. A step-by-step explanation of how to draw the C2Cl2 Lewis Dot StructureFor the C2Cl2 Lewis structure calculate the total number of valence electrons for th. To know the lewis structure it is vital to find.
It is a colorless oily liquid with a chloroform -like odor. Lewis Structures Names Data Sheets. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
11-Dichloroethane is a chlorinated hydrocarbon. This is the C2H2Cl2 Lewis structure. It is also a grain fumigant and has limited use as a solvent for plastics oils fats paint and varnishes.
7 rows 12-Dichloroethane-d4 C2H4Cl2 CID 12197860 - structure chemical names physical and. It is not easily soluble in water but miscible with most organic solvents. For this compound there is one molecule of Carbon two molecules of Hydrogen and two molecules of Chlorine.
Carbons the least electronegative and well put that in the center and we know Hydrogens always go on the outside. Carbon has 4 valence electrons two Carbons. Large volumes of 11-dichloroethane are manufactured with annual production exceeding 1 million pounds in.
Determine the central atom in this molecule. Models are useful because a Lewis structure as it is drawn on paper is not a good three-dimensional representation of a molecule. Loose Leaf for Organic Chemistry 5th Edition Edit edition Solutions for Chapter 1 Problem 9P.
How many shared electron pairs are there in the Lewis structure of C2H4Cl2. In C 2 H 4 due to small electronegativity difference between C and H the C-H bond is slightly polar but the molecule is symmetrical around its centre line which results in even charge distribution and makes the molecule non-. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule.
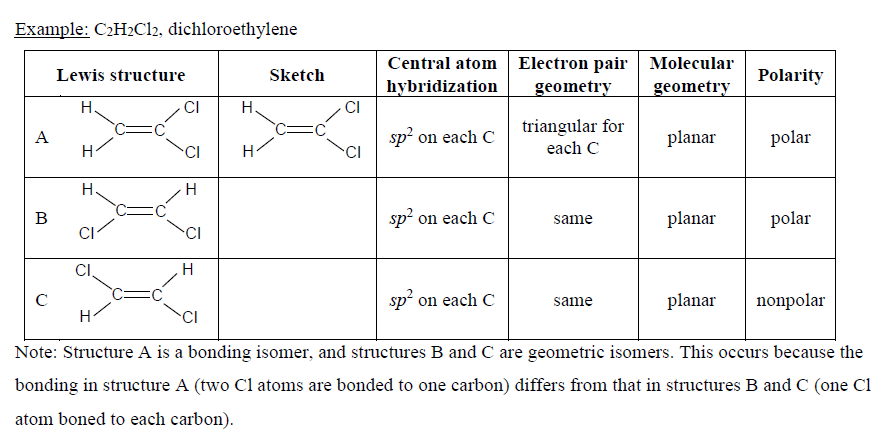
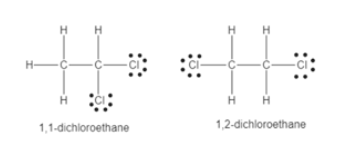
Draw structures for all constitutional isomers with the following molecular formulas. 11-Dichloroethane is used in the manufacture of high vacuum rubber and silicone grease. Draw the Lewis structure for the molecule.
Dichloroethane 12-dichloroethane 107-06-2 ethylene dichloride ClCH2CH2Cl.
4 2 Lewis Structures Problems Chemistry Libretexts

C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube
Answered Draw Lewis Structures For Each Bartleby
Solved Draw Lewis Structures For Each Molecular Formulaa C2h4cl2 Chegg Com
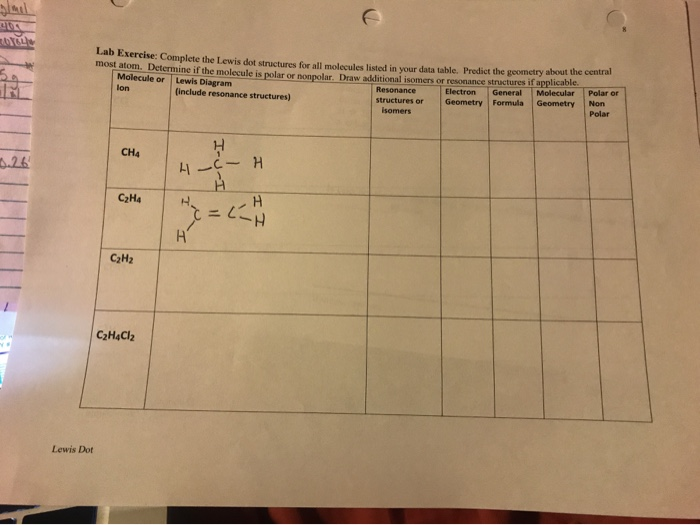
Molecule Or Lewis Diagram Include Resonance Chegg Com

C2 2 Lewis Structure How To Draw The Lewis Structure For C2 2 Acetylide Anion Youtube
What Is The Molecular Geometry Of C2h6 Quora
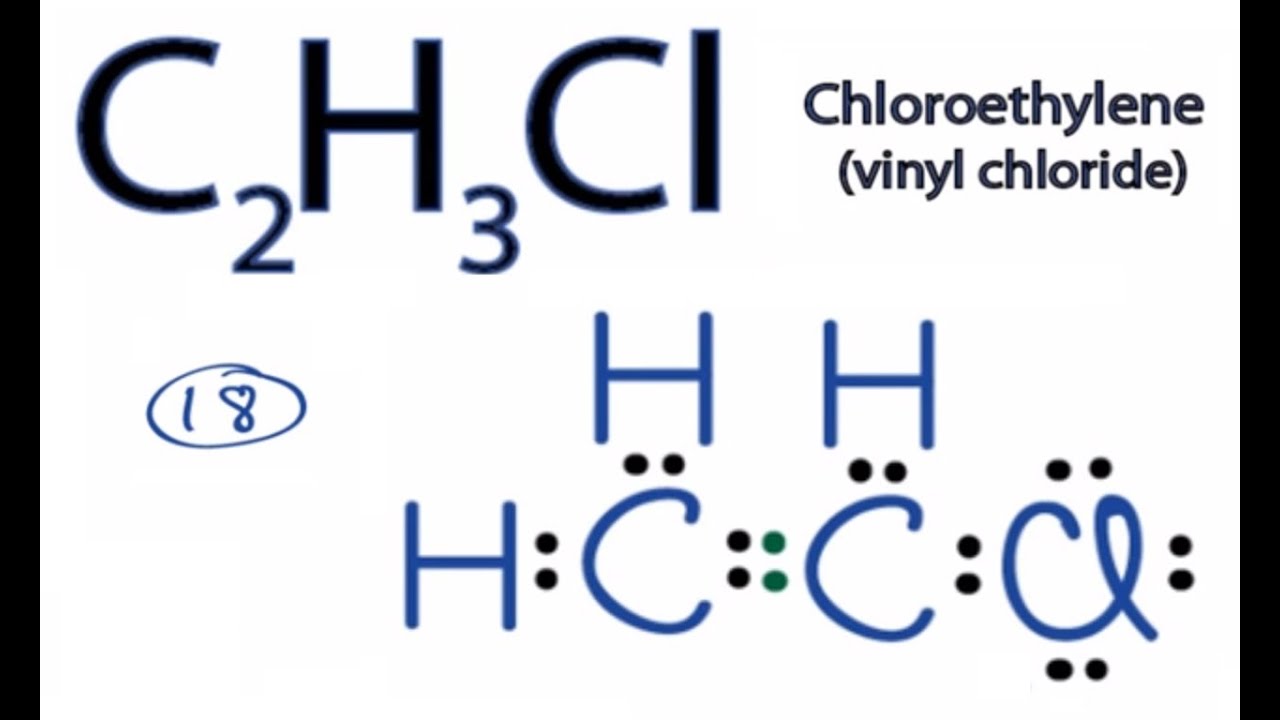
C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

Draw A Lewis Structure Of Formaldehyde Lewis Chemistry Draw
1 Choose One Column Of Compounds From The Table Chegg Com
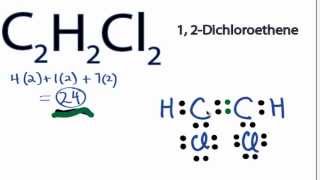
C2h2cl2 Lewis Structure How To Draw The Electron Dot Structure For C2h2cl2
1 1 Dichloroethane C2h4cl2 Chemspider
4 2 Lewis Structures Problems Chemistry Libretexts
Fdtc Chemistry Web Site Chm 111 Valence Bond Theory 1 R5 By Charles Taylor Charles Taylor Fdtc Edu
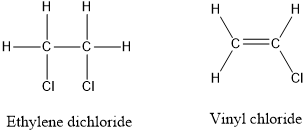
Solved Ethylene Dichloride C2h4cl2 Is Used To Make Vinyl Chlori Chegg Com

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Illustrated Glossary Of Organic Chemistry Kekule Structure
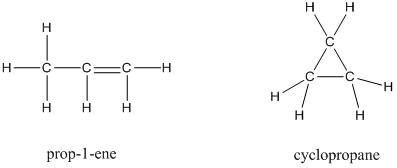
Solved Draw Lewis Structures For Each Molecular Formulaa C2h4cl2 Chegg Com