Hno3 Lewis Structure Molecular Geometry
The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. CS2 Lewis Structure Hybridization Molecular Shape and Polarity SO2 Lewis Structure Hybridization Molecular Geometry and MO Diagram H2CO3 Lewis Structure Molecular.

Draw A Lewis Structure For Nitric Acid The Hydrogen Atom Is Attached To One Of The Oxygen Atoms Brainly Com
Nitrous acid has a relatively lower percentage of oxygen than nitric.

Hno3 lewis structure molecular geometry. We write the Lewis structure. Solutions To Molecular Geometry Bonding. The central Sn atom is bonded to the three Cl atoms.
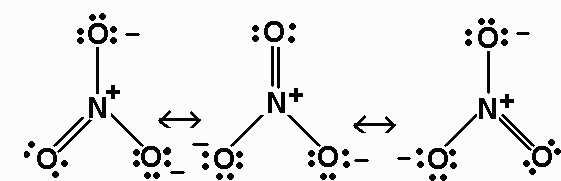
The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. 3 ion is. The Lewis structure of HNO₃ shows that it is a resonance hybrid of two structures.
The overall formal charge in ClO- is -1. Used to predict the molecular geometry. Describe the molecular geometry of HNO3.
3 Determining Molecular Shape 10700 1009 PM VSEPR- Procedural Steps 1 Determine the Lewis Structure. Has four electron domains around it. The lewis structure of nitric acid involves one hydrogen which possesses one valence electron nitrogen contains five and oxygen contains six valence electrons.
A Valence electrons for each atom in the structure. 16 bonding and non-bonding electron pairs. BrCl5 HNO3 POCl3 Question.
The bond angle about the Cl atom in d. Another way of looking at molecular geometries is through the AXE method of electron counting. B The Lewis structure for the SnCl.
For questions 2-8 write the BEST Lewis dot structure for each molecule being sure to give the electronic geometry molecular geometry hybridization of the central element polarity and bond angle around the central element. The central carbon atom is still joined to two other atoms. 16 8 Molecular Structure And Acid Base Behavior Chemistry.
And has one nonbonding pair. Be sure to use the number of available valence electrons you found earlier. What S The Lewis Of H2so3 Sulfurous Acid.
The HNO3 Lewis structure has 24 valence electrons. Note that when nitric acid dissociates. By signing up youll get thousands of step-by-step solutions to your homework questions.
It is a weak acid and exists only in solution form in the form of nitrite salts NO2-. Name the electron-group geometry. The first two have an NOH and a list how many electron groups are around the atom.
Draw the Lewis structures of the HNO2 HNO3 H2S PH3 CH3F and HCCH acetylene. HNO_3aq H_2Ol rarr H_3O NO_3- Visit BYJUS to understand the properties structure and uses of Magnesium Chloride. Electron-domain geometry is tetrahedral Table 91 with one of the corners occupied by a nonbonding.
Identify the central atom s in all of these structures Hint. After determining how many valence electrons there are in HNO3 place them around the central atom to. The N atom in HNO3 has SP2 hybridization and the O atom has SP3 hybridization.
The hybridization of chlorine and oxygen in the ClO- the molecule is Sp 3. In the Lewis structure of HNO3 the molecule is formed due to the hybridization of two orbitals. Looking at the positions of other atomic nuclei around the central determine the molecular geometry.
HNO 3 See Lewis Structure Tutorial H O N O O. Also what is the shape of Hocl. Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
Solved 1 Draw The Lewis Structures For Each Ac Acid Form. For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule. Draw the Lewis Structure.
Complete the octet for the central atom with the remaining electrons. HNO2 or Nitrous Acid comes under the category of monoprotic acids acids that donate one proton during dissociation. B Determine the atomic sequence the number of bonds remaining electrons c Write Lewis structure with each atom obeying the octet rule Example.
Drawing the Lewis Structure for HNO After determining how many valence electrons there are in HNO3 place them around the central atom to complete the octets. Hocl molecular shape - You can only upload photos smaller than MB. Drawing the Lewis Structure for HNO 3.
The bond angle in ClO- is 180º. The oxygen atoms will also have two p orbitals which will accommodate lone pair of electrons. The total valence electron available for the Hypochlorite ClO- lewis structure is 14.
In addition Lewis structures do not tell you about the 3-dimensional geometry of a molecule. Nitrogen has a steric number of 3 while the oxygen atom in the OH ion has a steric number of 4. Therefore the Sn atom.
3 H P 5 24 O 32 electrons ie. H O 3 P O The given Lewis structure distributes 5 bonding electron pairs about the central phosphorus atom. B write the electron group geometry c write the molecular geometry d determine if the molecule is.
ClO- is a non-polar molecule as it has a symmetrical structure and zero dipole moment. What are the different basic molecular shape. After bonding there are six hybrid orbitals in HNO₃.
Sulfurous Acid H2so3 Pubchem. The electron geometry is trigonal planar. The N atom has steric number SN 3.
HNO2 Lewis Structure Molecular Geometry Hybridization and Polarity. H2co3 Lewis Structure How To Draw The Lewis Structure For Carbonic Acid. This is a pattern seen with many acids.
The HNO3 Lewis structure has 24 valence electrons.

Resonance Structures For Hno3 Nitric Acid Youtube
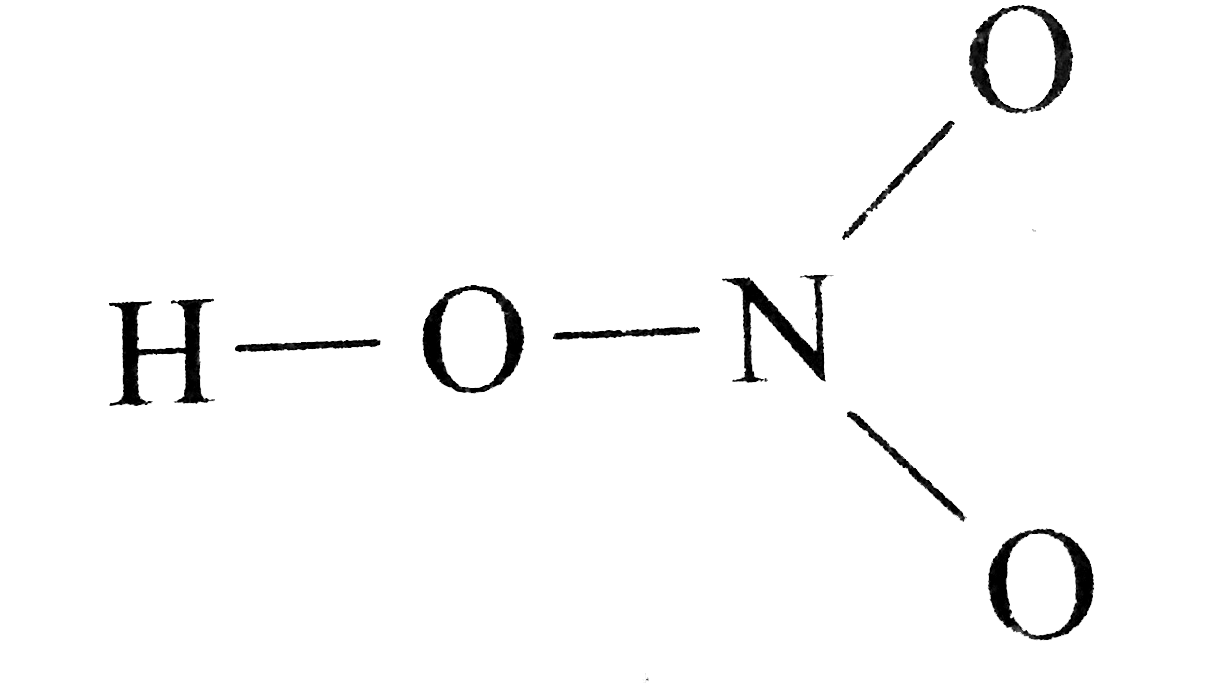
Draw The Lewis Structure Of Nitric Acid Hno 3
In Lewis Structure Of Hno3 Does Nitrogen Share Two Electrons With Two Of The Oxygens Do Those Oxygens Share Or Not Share Electrons With The Nitrogen Quora

How To Determine The Molecular Geometry Of Hno3 Quora

The Lewis Structure Of Hno3 Chemistry Stack Exchange

H2co3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
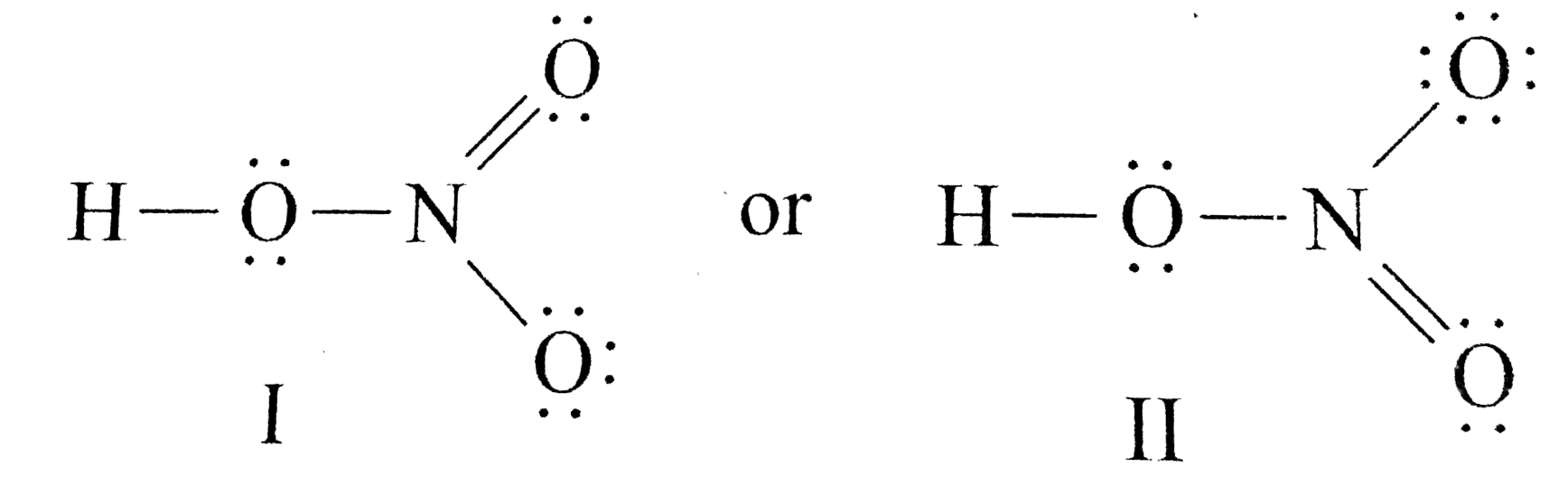
Draw The Lewis Structure Of Nitric Acid Hno 3

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

How To Determine The Molecular Geometry Of Hno3 Quora

Hno2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

1 Draw The Lewis Structure Of Nitric Acid Hno 3 That Minimizes Formal Charges Assign Lone Pairs Radical Electrons And Atomic Charges Where Appropriate 2 Calculate The Electrons Required Er Study Com

Describe The Molecular Geometry Of Hno3 Study Com
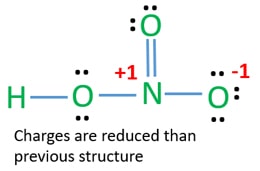
Hno3 Nitric Acid Lewis Structure

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Lewis Structure For Hno3 And State Its Molecular Geometry Is It Polar Or Nonpolar Study Com

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
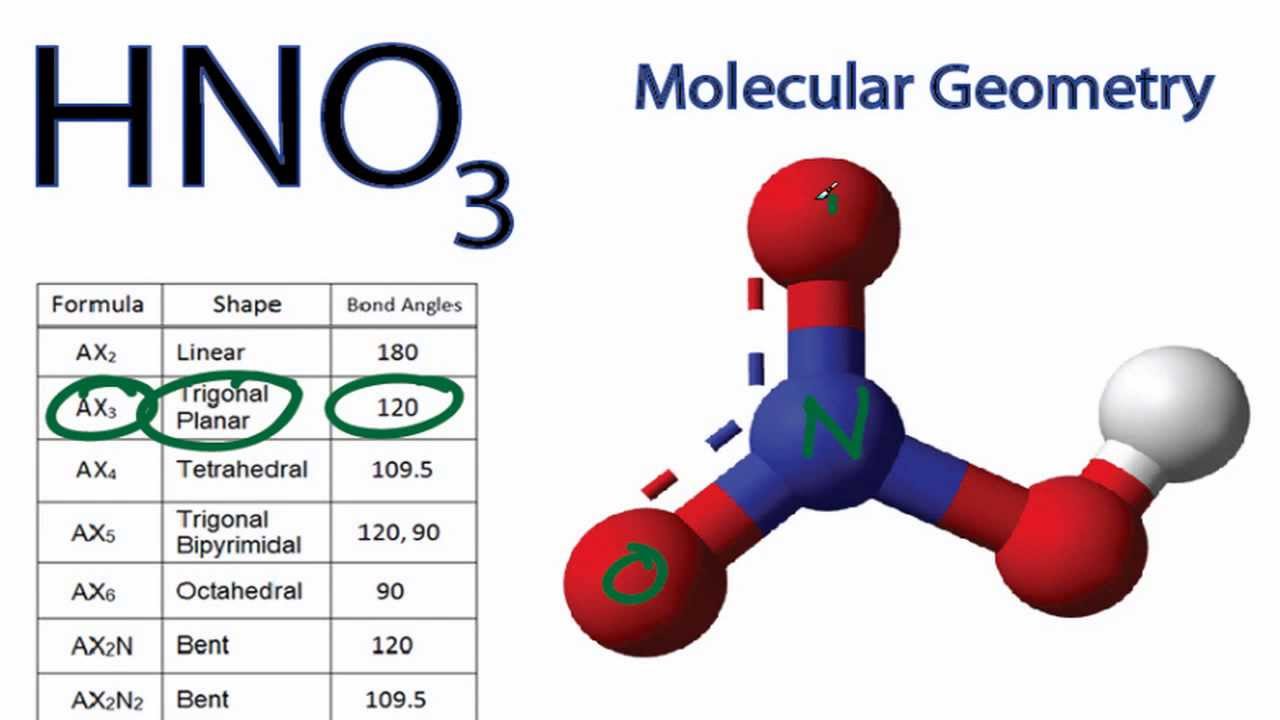
Hno3 Molecular Geometry Shape And Bond Angles Youtube