What Is The Molecular Geometry Of Ncl3
Its electron shape would be tetrahedral that is when you count the lone pairs of electrons as bonds themselves. It is polar because its charges are distributed asymmetrically and its geometric shape is asymmetricalPolar Molecules.
Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry
The molecular geometry of NCl3 is 1.

What is the molecular geometry of ncl3. According to the VSEPR theory the molecular geometry of NCl3 is trigonal pyramidal and electron geometry is tetrahedral because nitrogen being pentavalent has Sp³ hybridization with 5 valence electrons in its outermost shell and it makes three bond pairs one with each chlorine atom. In NCl3 N has one lone-pair of electrons. Use VSEPR theory to predict the molecular geometry of nitrogen trichloride NCl3.
An NCl3 molecule would be a trigonal pyramidal because it has one center N atom with 3 Cl surrounding it but also a lone pair of electrons on the top which bends the molecule downward forming a trigonal pyramidal. PCl3 Molecular Electron Geometry Lewis Structure Bond Angles and Hybridization. Is the molecular shape of the NCl3 molecule straight line linear pyramidal bent trigonal planar or tetrahedral.
None of these 3. Our mission is to help you succeed in your Chemistry class. If so feel free to rate me ath.
Chemistry questions and answers. An NCl3 molecule would be a trigonal pyramidal because it has one center N atom with 3 Cl surrounding it but also a lone pair of electrons on the. This problem has been solved.
Thus the electron-pair geometry is tetrahedral with three of the corners occupied by the bonding pairs of electrons. Get the detailed answer. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral.
The electron-pair geometry is trigonal-planar the molecular geometry is trigonal-planar. The electron geometry of this molecule will be tetrahedral and it will have eqrmsrmprm3 eq hybridization. What is the molecular geometry of ncl3.
The molecular structure of NCl3 is a trigonal pyramidal b none of these c octahedral d trigonal planar e bent. Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride NCl 3. Bent Calculate the work for the expansion of CO2 from 10 to 58 liters against a pressure of 10 atm at constant temperature.
Log in Sign up. The Correct Answer is. 19 Votes SOLUTION a The Lewis structure for the SnCl3-.
Learn how to draw a Lewis Structure and how to use it to assign a molecules shape geometryAre you taking a class with me. 7 rows NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and. 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3.
It is a volatile liquid that reacts with water and releases HCl gas. The molecular geometry of NCl3 is 1. The electron-pair geometry is linear the molecular geometry is linear.
A linear b trigonal planar c bent d tetrahedron e trigonal pyramid. What is the molecular geometry of ncl3. The central Sn atom is surrounded by one nonbonding electron pair and three single bonds.
This gives rise to a stable 120 degree angle between each of the bond-pair electrons. Boron in BF3 has no lone-pair electrons so the most stable arrangement of the bond-pair electrons would be where the repulsive energy is the least. Sign up to view answer.
2 or more lines of symmetry when you cut What is the molecular polarity of NCl3 and why. Free unlimited access for 30 days limited time only. The molecular geometry of the molecule will be trigonal pyramidal.
495 4304 Views. The skeleton structure is given below.

Hybridization For Ncl3 Description Of Hybrid Orbitals For Nitrogen Youtube

Lewis Structure Of Ncl3 Nitrogen Trichlorode Youtube

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep
What Is The Molecular Geometry Of Ncl3 Quora

Ncl3 Molecular Geometry Bond Angles Electron Geometry Nitrogen Trichloride Youtube

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Determine The Electron Geometry Eg And M Clutch Prep

Determine The Electron Geometry Eg And M Clutch Prep

File Ncl3 Dimensions Svg Wikimedia Commons

Hybridization For Ncl3 Description Of Hybrid Orbitals For Nitrogen Youtube

Draw The Lewis Structure For Ncl3 And Provide The Following Information A Number Of Electron Groups B Electron Pair Geometry C Bond Angle D Number Of Bonded Electrons E Molecular Geometry F

Homework 10 30 Points 1 Draw The Lewis Structure Chegg Com

Is Ncl3 Polar Or Nonpolar Techiescientist
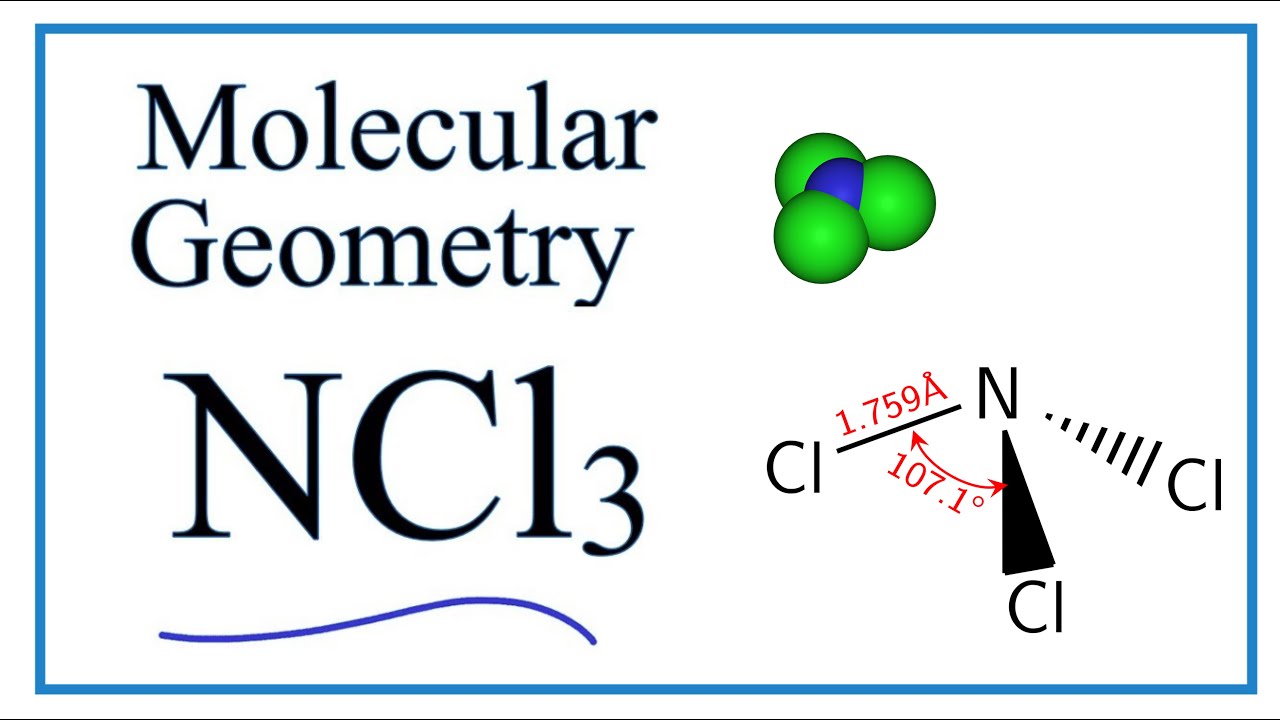
Ncl3 Molecular Geometry Shape And Bond Angles Youtube
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube