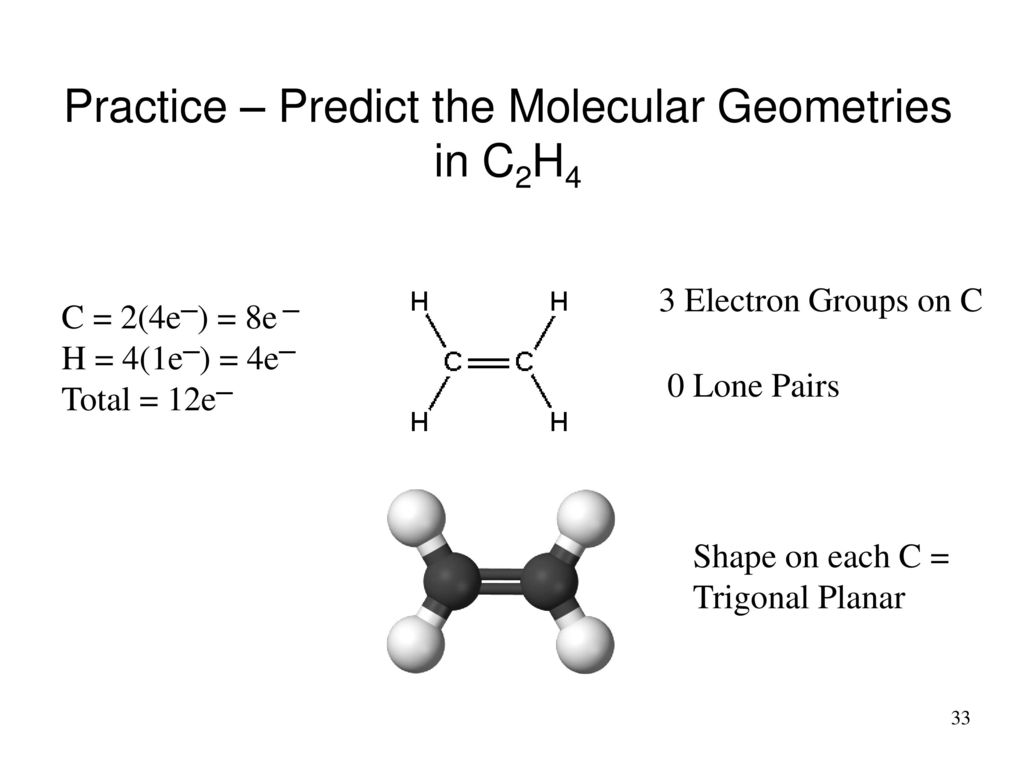
C2h4 Molecular Geometry Shape
See the title structure below. Well determine the N2H4 molecular geometry with respect to the Nitrogen on the right the other Nitrogen atom will have the same shape since they are symmet.
What Is The Hybridization And Bond Angle Of A C2h4 Molecule Quora
As e ach carbon in the C2H4 molecule has Sp² hybridization and with two hydrogens it makes the structure look like a triangular planar which is two-dimensional.

C2h4 molecular geometry shape. Read More About Hybridization of Other Chemical Compounds. If these are all bond pairs the molecular geometry is tetrahedral eg. A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the.
A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. If there are two bond pairs and two lone pairs of. As mentioned above the molecule has a tetrahedral geometry without any lone pairs.
This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. A quick explanation of the molecular geometry of CH4 including a description of the CH4 bond anglesLooking at the CH4 Lewis structure we can see that there. C2H6 Molecular Geometry Shape and Bond Angles.
And thank you for watching. The molecular geometry of C2H4 is trigonal planar and its electron geometry is also trigonal planar according to VSEPR Valence shell electron pair repulsion theory. In its most stable state the two Carbon atoms act as the central atoms in the structure.
Molecular Geometry of BF3. However the electron clouds that are on both the Carbon atoms will repel each other. If playback doesnt begin shortly try restarting your device.
So this is the lowest structure of C two H four in which there is a double bond between two carbon atoms and the carbon atoms are forming single bonds with two hydrogen atoms over here. Make sure to subscribe to our channel. Therefore the molecular geometry of C 2 H 4 is Trigonal Planar.
Flat but I cant see anything from the angle. So I hope this video helps you to understand this and for more such videos on lowest structure molecular geometry Polarity of the molecules. Another reason is that the hydrogen-carbon bonds are nonpolar because of nearly the same electronegativity.
CH 4 Molecular Geometry. C 2 H 4 consists of two Carbon atoms surrounded by two Hydrogen atoms on each end. They say both shape and angle here.
The molecular geometry of C2H4 is trigonal planar and its electron geometry is also trigonal planar according to VSEPR Valence shell electron pair repulsion theory. This means that the. In C 2 H 6 1s orbital and three p-orbitals px py pz take part in hybridizationThere is a formation of four sp 3 hybridized orbitalsDuring the hybridization of ethane four identical bonds are formed in a perfect tetrahedral geometry.
C2H4 Molecular Geometry And Bond Angles. As a result the dipole of the molecule of Ethylene turns out. C2h4 Molecular Geometry What is the shape of the molecule and the binding angle of C2H4.
If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal eg. A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Four electron pairs are distributed in a tetrahedral shape.
C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o. Get an dea of the overall shape. Ethanol CH3CH2OH or C2H6O CID 702 structure the structural formulas the organization of the individual molecules in space are different between ethanol.
Ethylene C2H4 is nonpolar in nature because of the symmetrical linear geometrical shape. Jacobs PA On-Line Single Run Analysis of Effluents from a Fischer-Tropsch Reactor J. Lets quickly summarize the salient features of C 2 H 4.
What is the shape and geometry of C2H4. The molecular geometry of C2H4 is a trigonal planar with respect to carbon left and right. Many people are confused that C2H4 is tetrahedral but its not tetrahedral C2H4 has a trigonal planar shape.
These forces lead to the formation of a trigonal pyramidal shape for this molecule. They form double bonds with each other and covalent bonds with the. Note the Hydrogen atoms H should not have lone pair.

Wn C2h3f Lewis Structure Polar Or Nonpolar Bond Angle Molecular Geometry Hybridization

Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube

Of2 Molecular Geometry Oxygen Difluoride Youtube

C2h4 Molecular Geometry Shape And Bond Angles Youtube

Molecular Geometry Predicted By Vsepr Ppt Download

C2h6 Molecular Geometry Shape And Bond Angles Youtube

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Priyanka Author At Geometry Of Molecules Page 2 Of 15
Why Is An Ethene Molecule A Planar Molecule While Ethyne Is Linear Quora

Draw The Lewis Structure For Methane Ch4 Ppt Video Online Download

C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Molecular Geometry Predicted By Vsepr Ppt Download
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
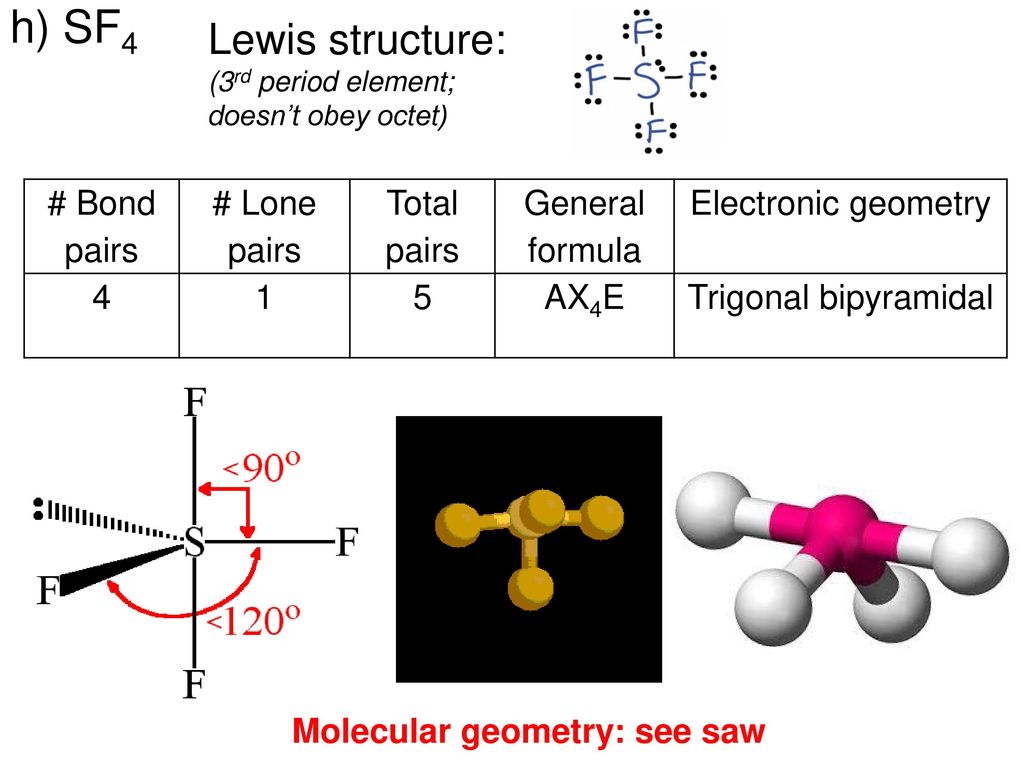
Molecular Geometry Predicted By Vsepr Ppt Download

Molecular Geometry Predicted By Vsepr Ppt Download