How Many Valence Electrons Does Clo3- Have
How many electrons and protons does a carbon atom have. Write the full and abbreviated electron configurations.
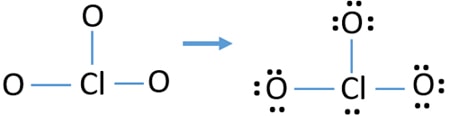
Lewis Structure Of Clo3 Chlorate Ion
The atomic number is the number of protons.

How many valence electrons does clo3- have. There are a total of 26 valence electrons for ClO 3-. Center atom of ClO3-ion To be the center atom ability of having greater valance is important. The Group 4 atoms have 4 valence electrons.
The answer to the question can help you on your way to understanding more about chemistry and its elements. The Group 3 atoms have 3 valence electrons. For example oxygen has six valence electrons two in the 2s subshell and four in the 2p subshell.
How many valence electrons does platinum have. Given this how many electrons are in each element. Valence electrons are the electrons in the outermost shell or energy level of an atom.
See the Big List of Lewis Structures. In this blog post we will be discussing how many valence electrons does Cl have. How many valence electrons does platinum have.
It acquires one e. The answer to the question is six. As a general rule a main group element except hydrogen or helium tends to react to form a s2p6 electron configuration.
How many valence electrons does californium have. Remember to put brackets around the Lewis structure along with a negative sign to show that it is an ion. The Group 2 atoms have 2 valence electrons.
Similar to other elements too the users can find out the valence electrons. That is the number of protons in the carbon is 6. ClO3-there are 26 valence electrons so total pairs of electrons are 13.
You may not know what that means but its important to understand how many electron orbitals are available for an element. Now if we talk about the element lead or Pb then there are four 4 electrons on the outer shell or the 6 th shell therefore the number of valence electrons that lead has is 4. There are 2 types of elements in the periodic table.
This tendency is called the octet rule because each bonded atom has 8 valence electrons including shared electrons. There are 18 electrons. Nitrogen has a total of 5 valence electrons so doubling that we would have a total of 10 valence electrons with two nitrogen atoms.
How many valence electrons are present in the electron configuration. Helium atoms do not form ions. The total number of valence electrons is 5611.
In model 1 you can only see. So number of valence electrons 718. The nucleus is located in the center of the atom.
When we say or talk about the valence shell electrons it could be found out as the total number of electrons that are there in the outer shell of any molecule or an atom. The atomic number of carbon is 6. How Many Valence Electrons Does Lead have.
This makes chlorines valence shell incomplete and the atom attracts loose electrons to this shell to complete it so the atom can be stable. Helium atoms do not usually do this. I am stuck on my homework and missing deadline.
Please help me in solving this I will pay. By signing up youll get thousands of step-by-step solutions to your homework. Metals are the elements which have a tendency to loose electrons and thus they form cations.
During radioactivity some of the particles emitted consist of 2 neutrons 2 protons and 2 electrons. Chlorine can show valence 7. It is a simple alpha particle called helium particle.
Sodium will loose 1 electron to form ion. How Many Valence Electrons Does Cl Have Number Of Valence Electrons In Cl April 23 2021. Therefore no matter how electrons are shared between the nitrogen and oxygen atoms there is no.
The Group 1 atoms have 1 valence electron. Electrons equal to protons are located in a circular shell. Protons and neutrons are located in the nucleus.
Ions are charged when they lose NS electrons. Magnesium is classified as a metal. Cl has a valence of six which means.
The valence electron of lithium is one. How many valence electrons does magnesium have. 2 Day Business Management Masters.
Magnesium has just two valence electrons and 12 electrons. How many valence electrons does nitrogen have. The abundance of chlorine-35 is 75 and the abundance of chlorine-37 is 25.
How many valence electrons does cl have. In this regard can nitrogen have 8 valence electrons. How Many Valence Electrons Does Helium Have How many valence electrons are there in helium ion.
Thus it has 7 valence electrons. Lithium is called alkali metal. Lithium is the element of group-1 and the symbol is Li.

What Is The Number Of Valence Electron In A Chloride Ion Quora

Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube

Science Coverage Valency Of Lithium How Many Valence Electrons Do In 2021 Electron Configuration Electrons Chemistry
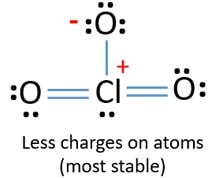
Lewis Structure Of Clo3 Chlorate Ion
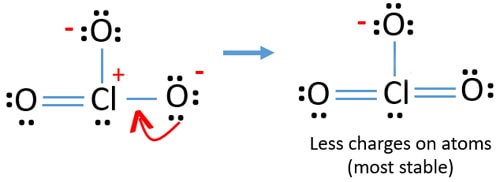
Lewis Structure Of Clo3 Chlorate Ion

Clo4 Lewis Structure How To Draw The Lewis Structure For Clo4 Perchlorate Ion Youtube

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
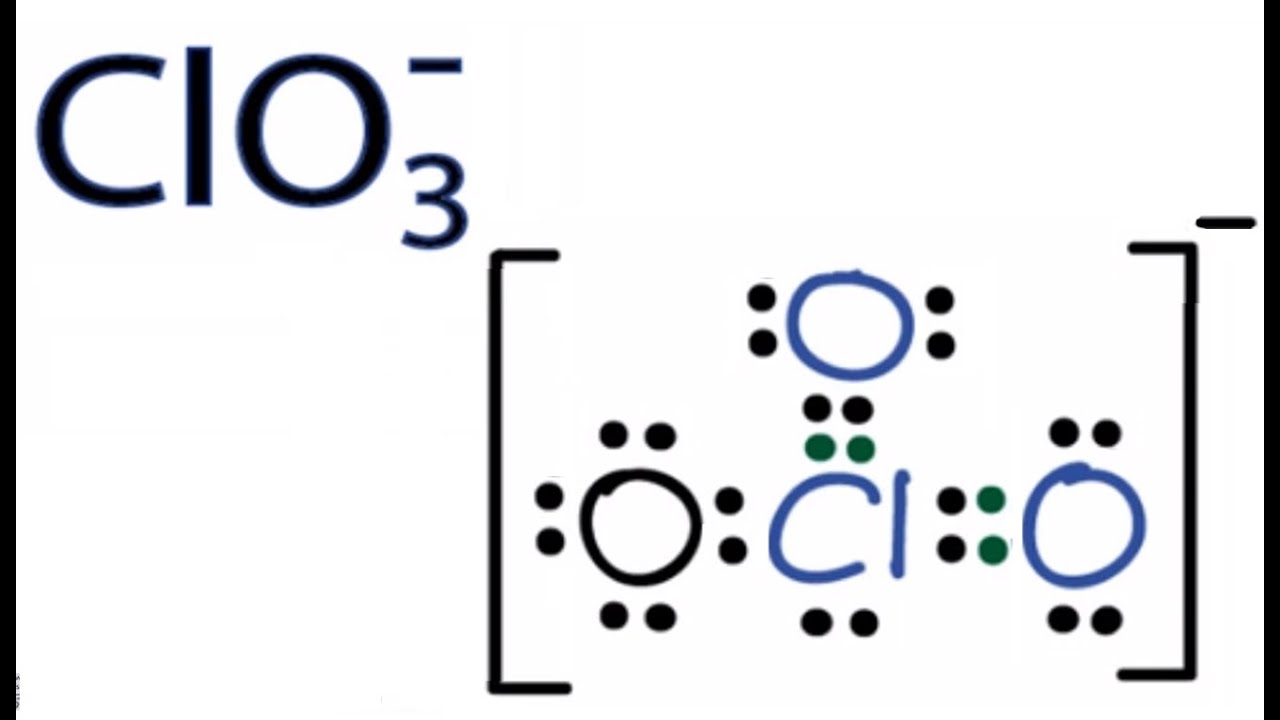
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube

Science Coverage Valency Of Cesium How Many Valence Electrons Doe In 2021 Electron Configuration Electrons Noble Gas

What Is The Lewis Structure For Ibr 2 Clutch Prep

126 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube Chemistry Classroom Science Chemistry Chemistry
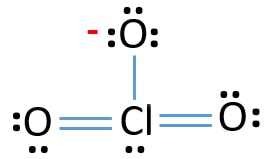
Lewis Structure Of Clo3 Chlorate Ion

How To Draw The Lewis Structure For Clo2 Chlorine Dioxide Youtube

How To Find Valence Electrons And Total Electrons Youtube Chemistry Lessons Teaching Chemistry Chemistry Help
How Many Valence Electrons Are There In Chlorine Quora

How Many Valence Electrons Does Chlorine Cl Have Valency Of Chlorine

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State
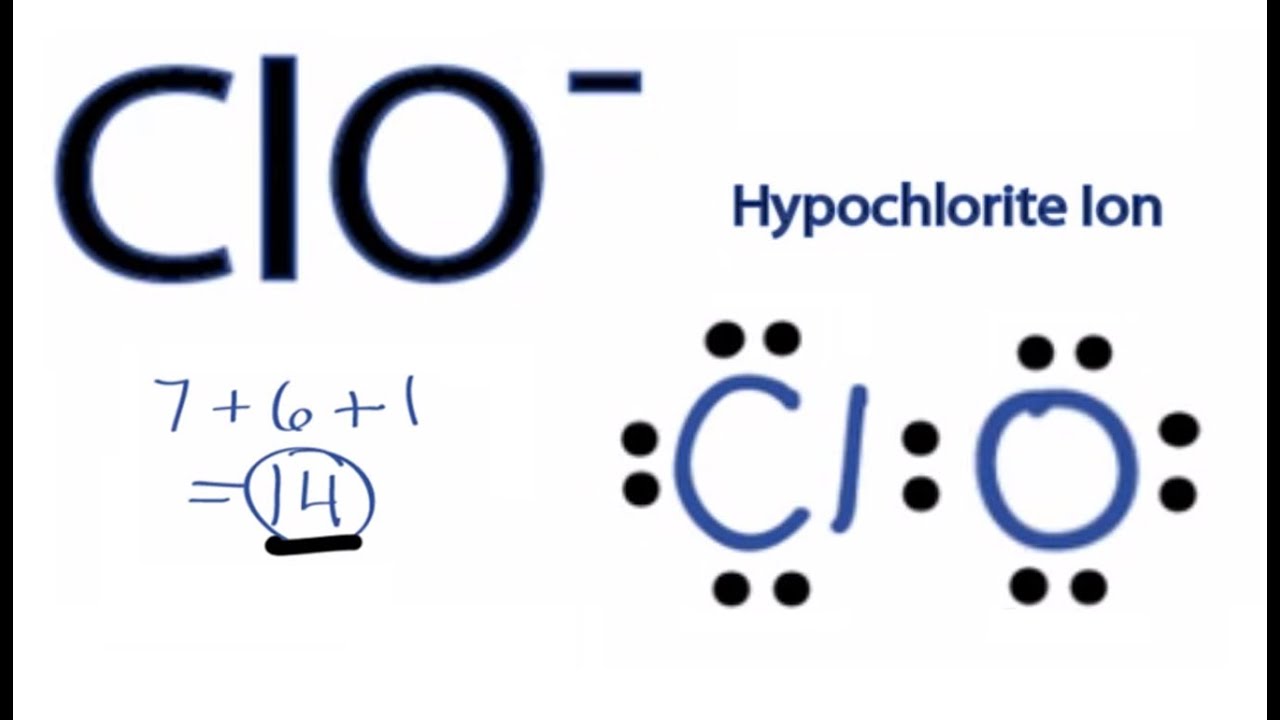
Clo Lewis Structure How To Draw The Lewis Structure For Clo Youtube