Is Bh3 A Lewis Base
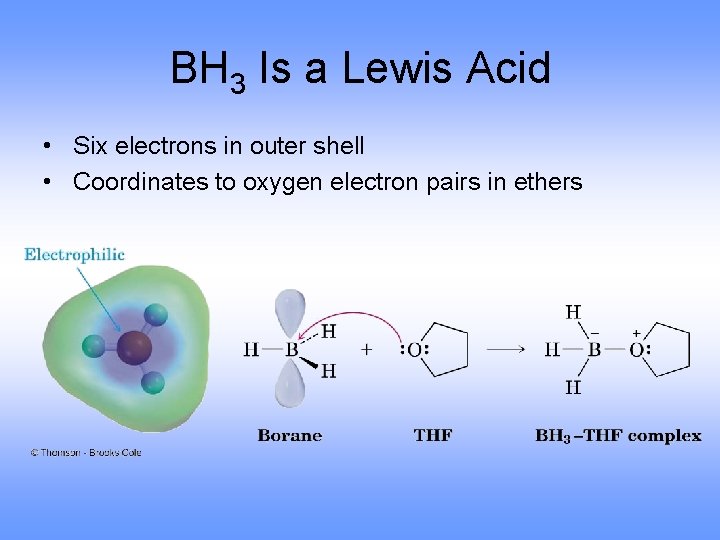
The Lewis structure of BH3 shows boron having only three bonds and no lone pairs of electrons allowing it to accept electrons from a donor. So BH3 can be regarded as a Lewis acid.
Illustrated Glossary Of Organic Chemistry Lewis Acid Base Adduct Lewis Acid Base Complex
The oxygen in CaO is an electron-pair donor so CaO is the Lewis base.

Is bh3 a lewis base. One type of exception to the octet rule are compounds with. The proton however. BH 3 is acting as a Lewis acid accepting a pair of electrons from CH 3 3 N to form a bond.
15 Is H a Lewis acid. Aluminum ion acts as a Lewis acid and accepts the electrons from water which is acting as a Lewis base. The Lewis base is CH 3 2 S and the Lewis acid is BH 3.
8 Why b2h6 is Hypovalent. Can BH3 act as a Lewis base. The Lewis structure of BH3 shows boron having only three bonds and no lone pairs of electrons allowing it to accept electrons from a donor.
A Lewis acid is defined as an electron-pair acceptor. People Also Asked Is bh3 a bronsted acid or base. The Lewis Acid accepts the electrons from the Lewis Base which donates the electrons.
Carbon accepts a pair of electrons so CO 2 is the Lewis acid. A Lewis acid with THF a Lewis base occurs when an oxygen lone pair forms an oxygen-boron bond. In various cases adduct violates the octet rule likewise the triiodide anion.
7 Which structure is similar to graphite. The reaction between the Lewis acid and base results in the formation of. 16 Is BeCl2 an.
13 Why BeCl2 is not a Lewis acid. The reaction product is a Lewis acid - Lewis base adduct because all of the reactant atoms are part of the product. 12 Why BaCl2 is not a Lewis acid.
14 Is BeCl2 an acid or base. Here F acts as an electron pair donor whereas BF 3 accepts the electron pair. Both BF4 and the BF3OMe2 are the Lewis base adducts of boron trifluoride.
Is BH3 an exception to the octet rule. Neither an acid nor a base. For example NH3 is a Lewis base because it can donate its lone pair of electrons.
Yes BH3 act as Lewis acid due to its incomplete octed it can easily accept lone pair from Lewis base their is only six electron in outer most shell of Boron in BH3 molecules. 11 Is B2H6 a Lewis acid. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct.
There is no leaving group. Yes BH3 act as Lewis acid due to its incomplete octed it can easily accept lone pair from Lewis base their is only six electron in outer most shell of Boron in BH3 molecules. This reaction features the formation of a coordinate bond between the fluoride anion F and boron trifluoride BF 3.
As in the reaction shown in Equation 821 CO 2 accepts a pair of electrons from the O 2 ion in CaO to form the carbonate ion. N H 3 M e N H 2 P y r i d i n e C O T. BH3 is acting as a Lewis acid accepting a pair of electrons from CH33N to form a bond.
Electron-deficient moleculessuch as BCl 3contain less than an octet of electrons around one atom and have a strong tendency to gain an additional pair of electrons by reacting with substances that possess a lone pair of electrons. The boron in BF3 is electron poor and has an empty orbital so it can accept a pair of electrons making it a Lewis acid. Trimethylborane is a Lewis.
Another case where Lewis acid-base theory can explain the resulting compound is the reaction of ammonia with Zn 2. Reaction of borane BH 3. 6 What is the structure of b2h6.
When B 2 H 6 is allowed to react with following Lewis bases then how many given Lewis bases form adduct through symmetrical cleavage of B 2 H 6. F P H 3 P F 3 M e 3 N M e 2 N H. I2 I I3 The colours variability of the iodine casts back the capability of variables of the solvent in order to make adducts with the Lewis acid I2.
Explain how during dimerization each BH3 acts as both lewis base and a lewis acid. A Lewis acid can accept a pair of electrons from a Lewis base. The reverse is not true not all Lewis acidbase are Bronsted acidbaseThe typical example is octet deficient boron compounds like BH3This is a Lewis acid because it will want to accept electrons to fill out its octet.
A Lewis base is defined as any species that can donate a pair of electrons and a Lewis acid is any species that can accept a pair of electrons. Is trimethyl aluminum Al CH 3 3 A. 9 What is the hybridization of b2h6.
Is bh3 a Lewis acid or base. A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. Keeping this in consideration is bf3 an acid or base.
5 Does BH3 exist. Bh3 A Lewis Acid Or Base Gastro-esophageal reflux disease often known as acid reflux disorder can be a physiological ailment that triggers regurgitation of your stomachs written content inside the esophagus and oral cavity. This helps explain the resulting hexaaquaaluminumIII ion.
What is the name of BH3. But it is not a Bronsted acid because it will never donate a proton. 10 Is bh3 sp3 hybridized.
BH3 is acting as a Lewis acid accepting a pair of electrons from CH33N to form a bond.

Pin On Nerdy Squirrel Transmissions

Lewis Acid Base Interaction In Ph3p Bh3 Protocol

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books
Chapter 5 Alkenes And Alkynes Ii Reactions Elimination
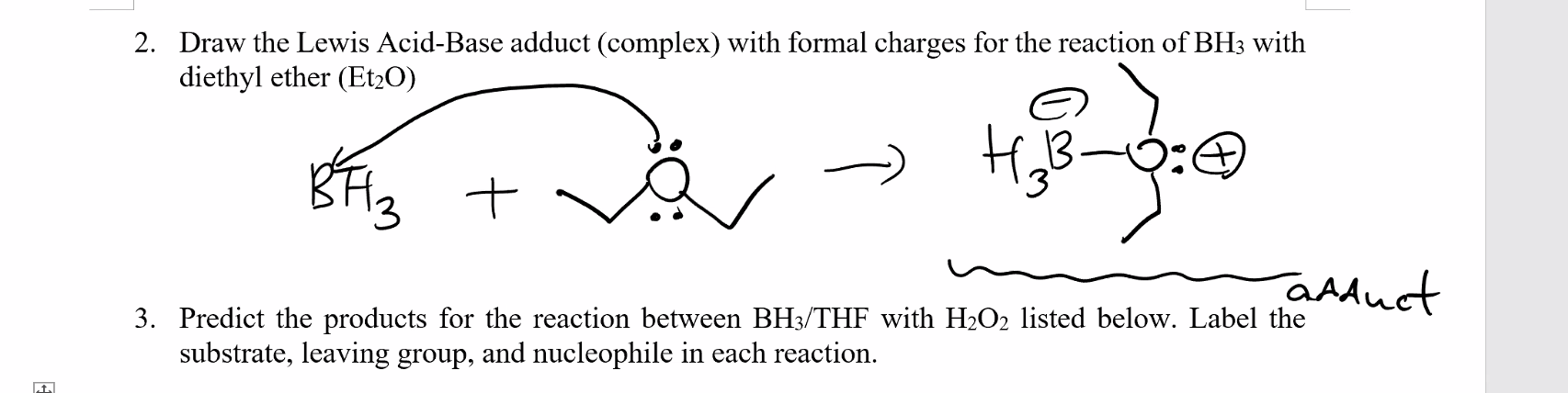
2 Draw The Lewis Acid Base Adduct Complex With Chegg Com

Borane Lewis Base Complexes As Homolytic Hydrogen Atom Donors Hioe 2010 Chemistry A European Journal Wiley Online Library

Which Of The Following Statements Is True A Bh3 Is A

Reagent Friday Bh3 Borane Organic Chemistry Teaching Chemistry Chemistry Help
Is Boron Trihydride Bh 3 A Base Socratic
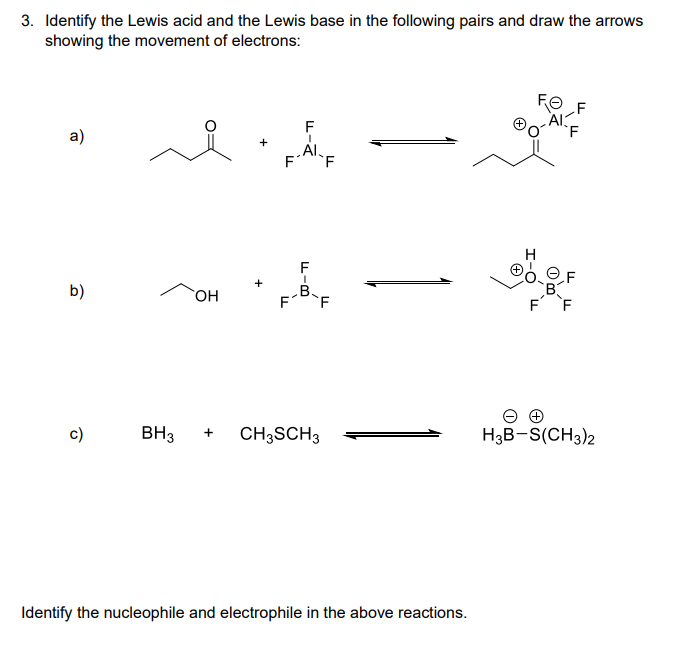
3 Identify The Lewis Acid And The Lewis Base In The Chegg Com
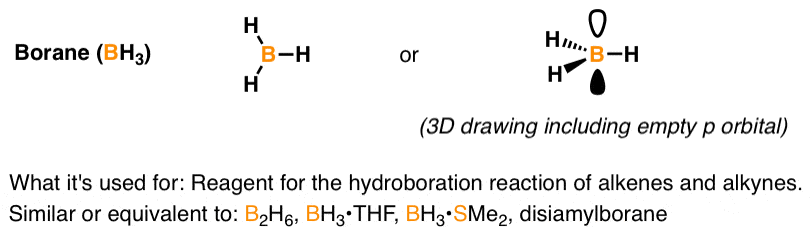
Reagent Friday Bh3 Borane Master Organic Chemistry

Azadiboriridine Borane A Non Classical Acid Base Adduct Paetzold 1990 Angewandte Chemie International Edition In English Wiley Online Library
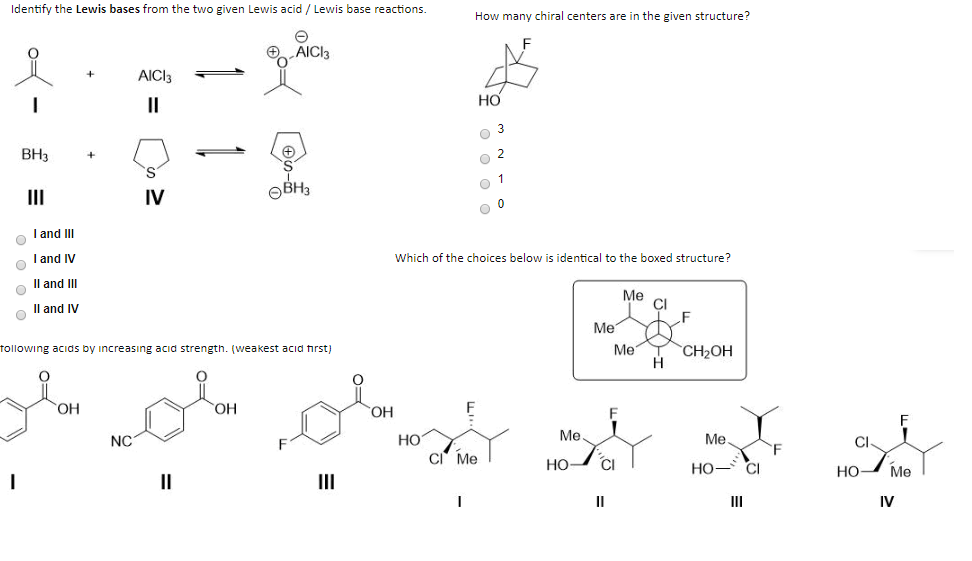
Identify The Lewis Bases From The Two Given Lewis Chegg Com

Lewis Acid Base Interaction In Ph3p Bh3 Protocol
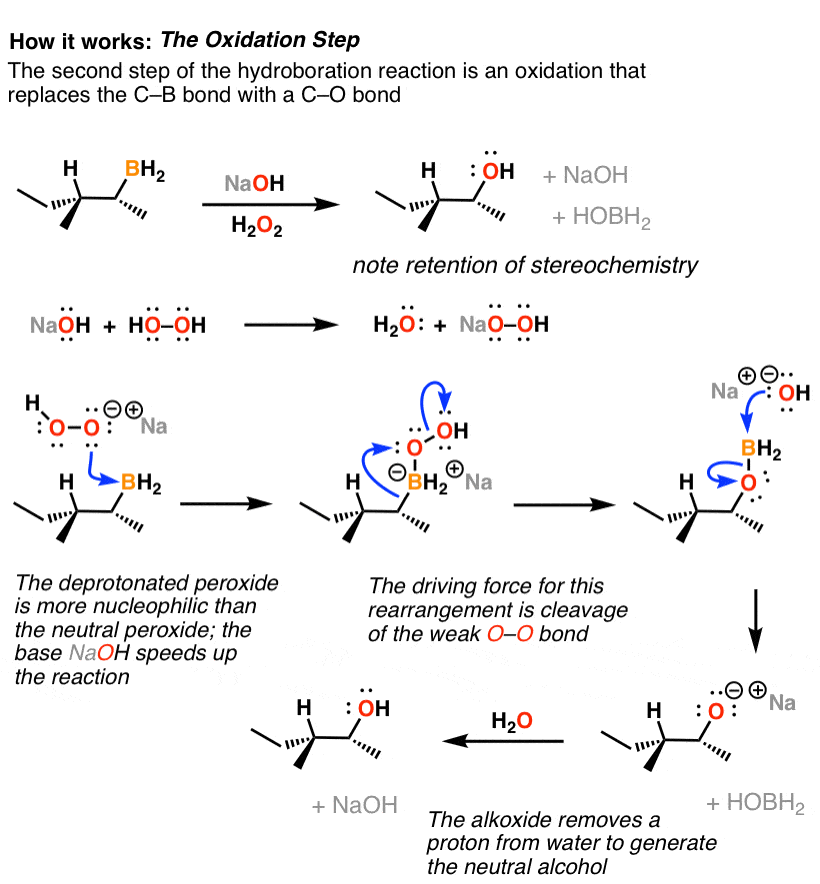
Reagent Friday Bh3 Borane Master Organic Chemistry
