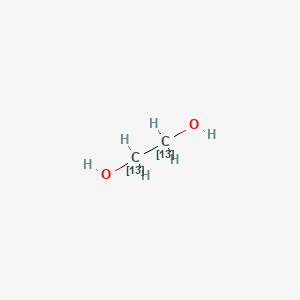
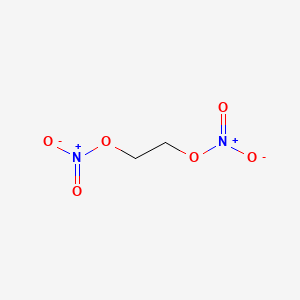
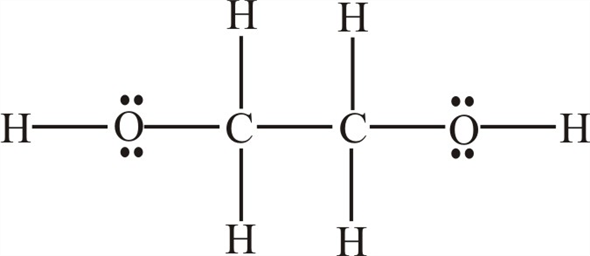
Lewis Structure For Ethylene Glycol
The Henrys Law constant for ethylene glycol is 600X10-8 atm-cu mmole 1. Ethylene glycol C 2 H 6 O 2 has one OH bonded to each carbon.
Ethylene Glycol Molecule Of The Month June 2018 Html Version
This information includes synonyms chemical formula and structure and identification numbers.
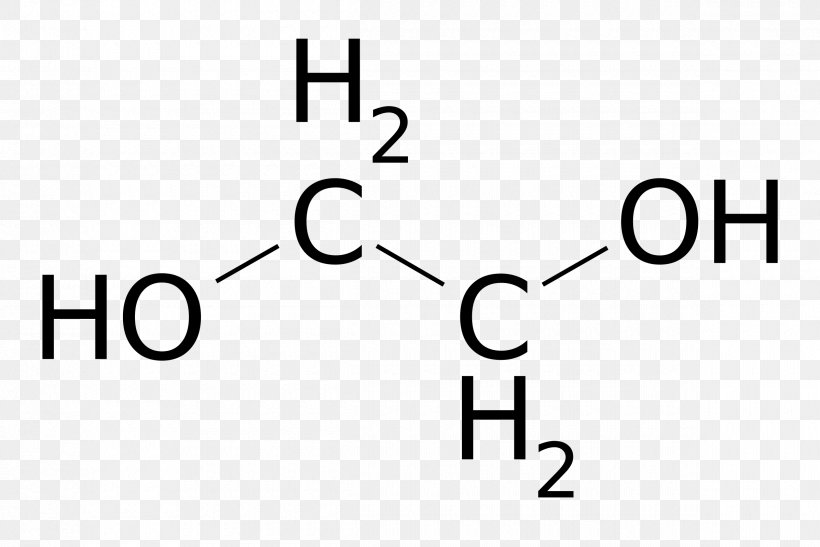
Lewis structure for ethylene glycol. Structural Formula C 2 H 6 O 2 ethylene glycol. 16 bonding and 4 non-bonding electrons E. Draw its Lewis structure and identify the sigma and pi bonds.
It is also known as Ethane-12-diol or Monoethylene glycol. In the molecule ethene both carbon atoms will be sp 2 hybridized and have one unpaired electron in a non-hybridized p orbital. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation.
Draw the Lewis dot diagram for the ethylene glycol molecule A. 42 PHYSICAL AND CHEMICAL PROPERTIES. 27 Ethylene Glycol Chemical Structure 28 29 Ethylene glycol is used as a de-icer and anti-icer antifreeze.
Top 2D structure of ethylene glycol. Ethylene glycol is also called ethane 12 diol. It is colourless and has a sweet taste.
Stearic acid ethyl ene ester 8CI. C 2 H 4 O H 2 O HOCH 2 CH 2 OH. 18 bonding and 4 non-bonding electrons B.
Also it is a 31 component in hydraulic brake fluid and inks and is used as a solvent. Ethylene Glycol Formula Structure. Information regarding the physical and chemical properties of ethylene glycol is located in Table 4-2.
These p-orbitals will undergo parallel overlap and form one latex sigma latex bond with bean-shaped probability areas above and below the plane of the six atoms. Problem 141 Medium Difficulty. B Draw the Lewis dot structure of chloroethane C 2 H 5 Cl.
Brief chemistry video describing ethylene glycol and one area its used. Antifreeze Research ethylene glycol an antifreeze-coolant to learn its chemical formula. In the Lewis structure of ethylene glycol HOCH2CH2OH how many electrons are bonding and how many are non- bonding.
Ethylene glycol is produced from ethylene ethene via the intermediate ethylene oxide. 18 bonding and 8 non-bonding electrons D. B Draw the Lewis dot structure of chloromethane C2H5cl c Chloroethane has a slightly higher molar mass than ethylene glycol but much lower.
A Draw the lewis dot structure of ethylene glycol. A Draw the Lewis dot structure of ethylene glycol. This Henrys Law constant indicates that ethylene glycol is expected to be essentially nonvolatile from water surfaces 2.
Ethylene Glycol based water solutions are common in heat-transfer applications where the temperature in the heat transfer fluid can be below 32 o F 0 o CEthylene glycol is also commonly used in heating applications that temporarily may not be operated cold in surroundings with freezing conditions - such as cars and machines with water cooled engines. The correct Lewis structure for ethene is shown below. Stearic acid ethyl ene ester.
It has no smell and is viscous. Ethylene Glycol is dihydroxy alcohol with a chemical formula C 2 H 6 O 2. Information regarding the chemical identity of ethylene glycol is located in Table 4-1.
Molecular Formula C 2 H 6 O 2. 22 bonding and 0 non-bonding electrons C. Monoisotopic mass 62036777 Da.
Ethylene glycols Henrys Law constant indicates that volatilization from moist soil surfaces is not expected SRC. Glycol distearate Wiki MFCD00053743 MDL number Octadecanoic Acid 1 2-Ethanediyl Ester. Average mass 62068 Da.
Bottom 3D structure of ethylene glycol Finally and not surprisingly now its formula can also be written in many ways. It is a deactivator for all pesticides used 30 before the crop emerges from the soil and in herbicides before or after the crop emerges. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript.
Density massvolume Mass of H number of moles relative molecular mass 1 1 1 g density 1624x1015 8065x10-16gL 8116 Ethylene glycol C2H6O2 has one OH bonded to each carbon. C Chloroethane has a slightly higher molar mass than ethylene glycol but a much lower boiling point 3. Species with the same structure.
HOCH 2 CH 2 OH. Octadecanoic acid 12-ethanediyl ester ACDIndex Name RG1690000. This organic compound is highly toxic.
Ethylene Glycol 13c2 C2h6o2 Pubchem

In The Lewis Dot Structure Of Ethylene Glycol Would One Hydrogen Atom Visibly Connect The Two Oxygen Atoms And If So Would One Line Connecting That Same Hydrogen Atom To One Of

Ethylene Glycol Structural Formula Molecule Ethylene Oxide Png 2400x1602px Ethylene Glycol Area Black Brand Chemical Compound

Ethylene Glycol Structural Formula Molecule Ethylene Oxide Angle White Png Pngegg

Ethylene Glycol Properties Uses Structure Britannica
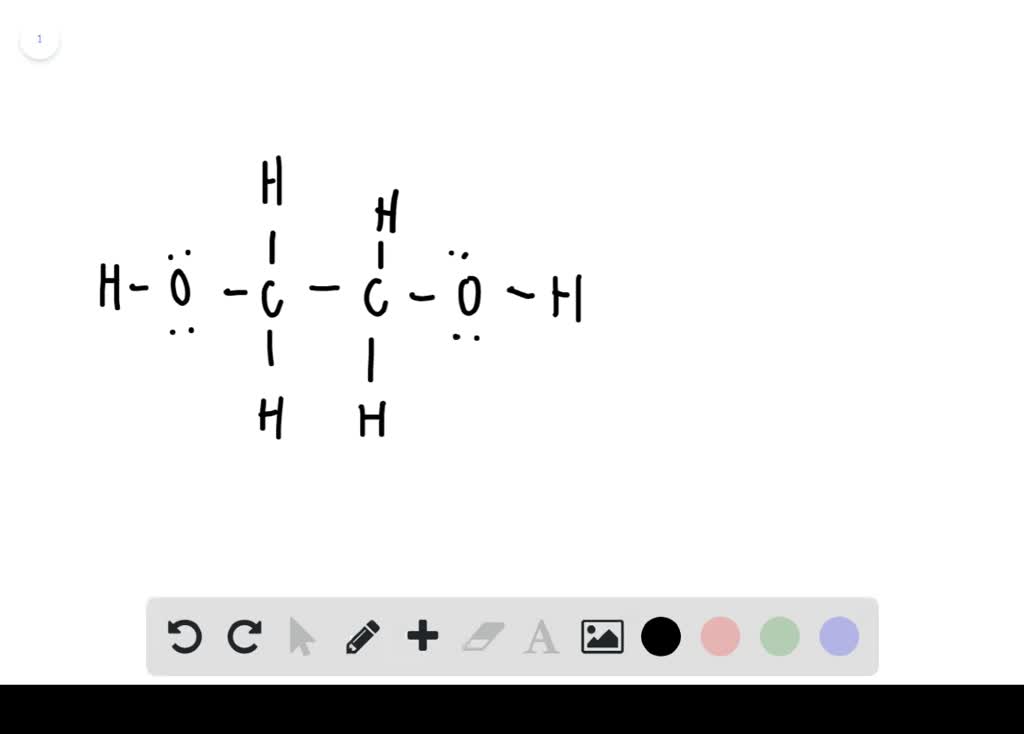
Solved Antifreeze Research Ethylene Glycol An Antifreeze Coolant To Learn Its Chemical Formula Draw Its Lewis Struct

Ethylene Glycol Propylene Glycol Diol Structural Formula Ethylene Glycol Dimethacrylate Angle White Text Png Pngwing
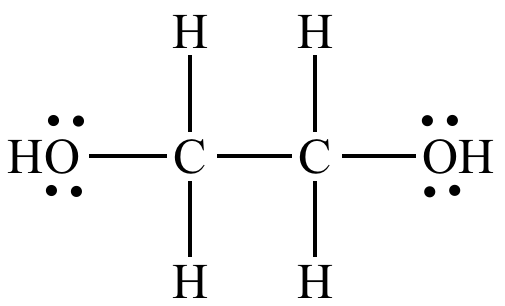
Illustrated Glossary Of Organic Chemistry Ethylene Glycol
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Ethylene Glycol C2h6o2 Chemspider
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Ethylene Glycol Dinitrate C2h4n2o6 Pubchem
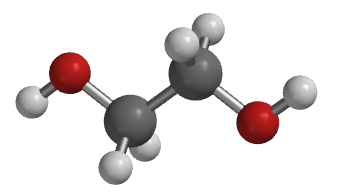
Illustrated Glossary Of Organic Chemistry Ethylene Glycol
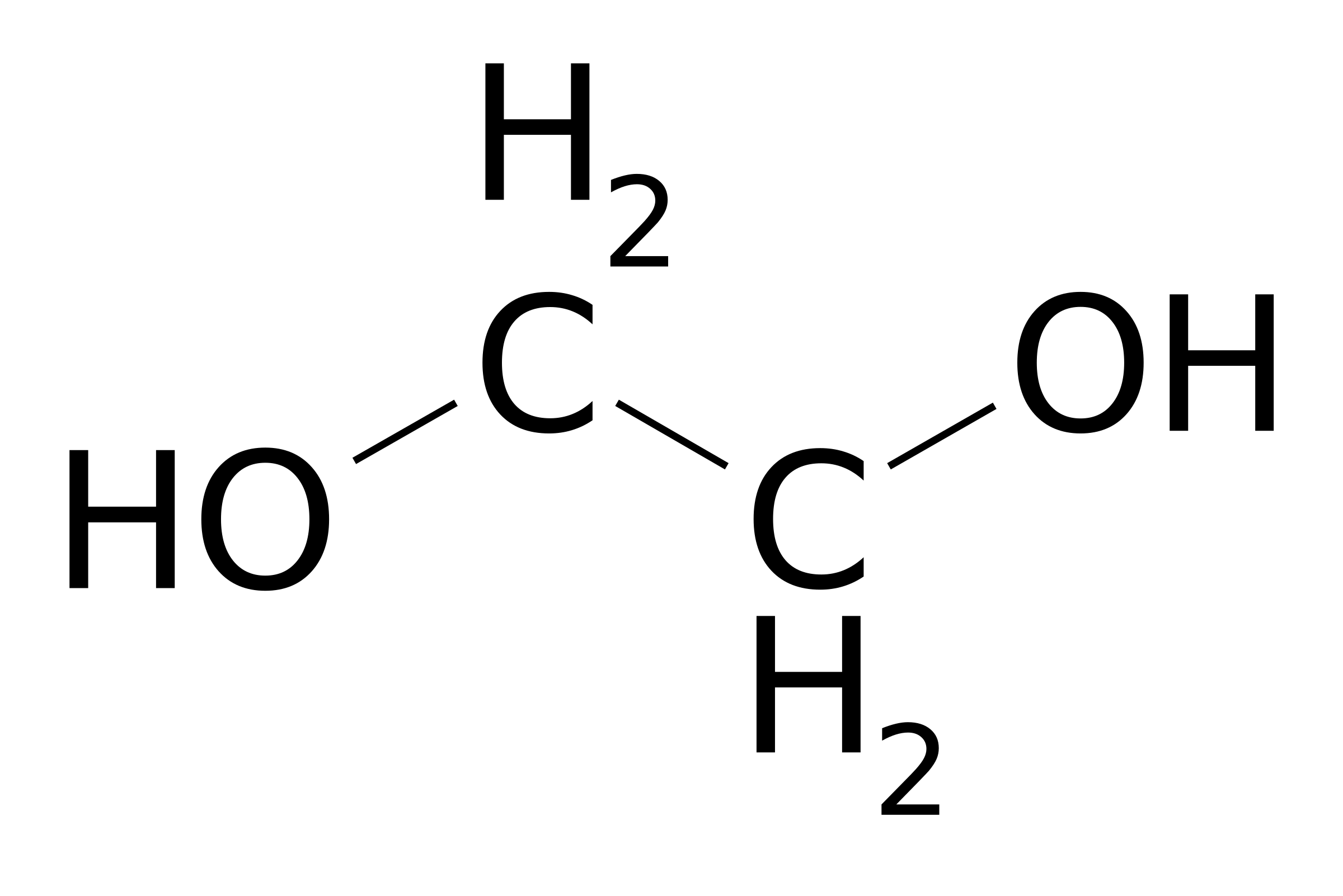
Is Ethylene Glycol Molecular Or Ionic Socratic
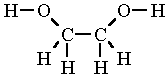
Ethylene Glycol Formula Structure
Solved Chapter 8 Problem 116ap Solution Masteringchemistry Standalone Access Card For Fundamentals Of General Organic And Biological Chemistry 7th Edition Chegg Com
Ethylene Glycol Molecule Of The Month June 2018 Html Version

In The Lewis Dot Structure Of Ethylene Glycol Would One Hydrogen Atom Visibly Connect The Two Oxygen Atoms And If So Would One Line Connecting That Same Hydrogen Atom To One Of

Ethylene Glycol Propylene Glycol Diol Structural Formula Ethylene Diurea Angle Text Png Pngegg