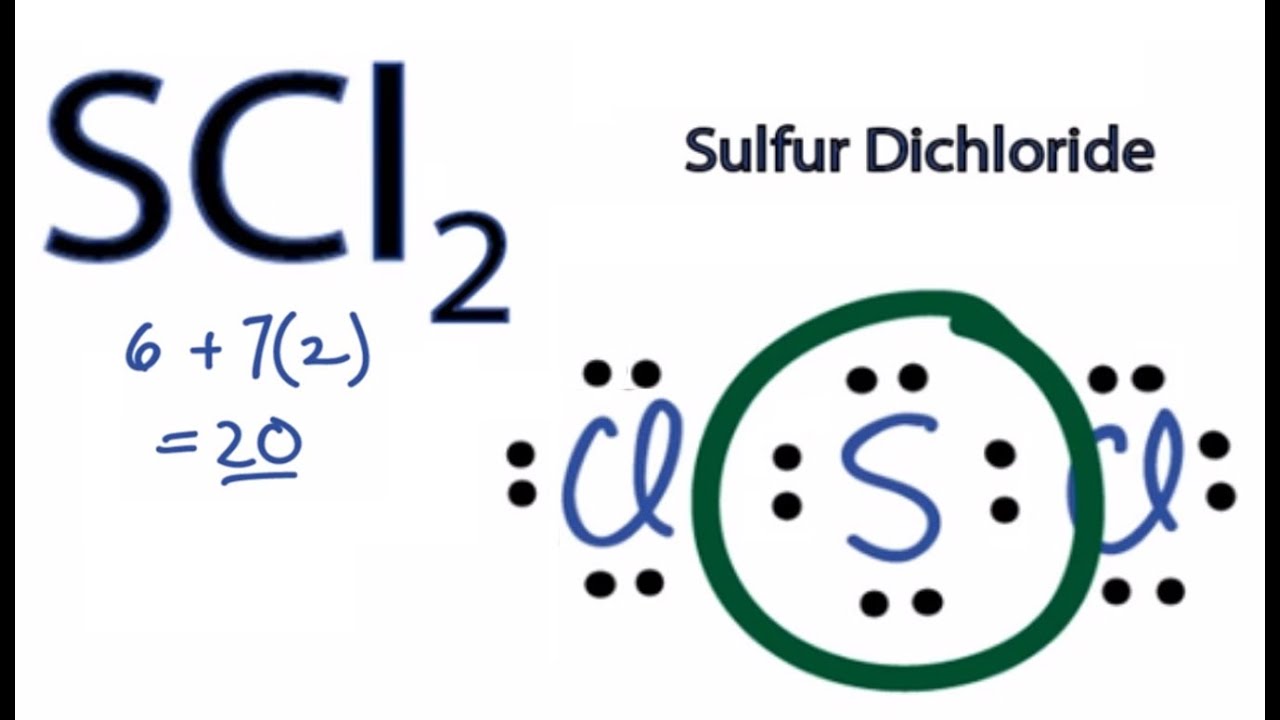
Lewis Structure For Sulfur Dichloride
SO2 Draw the Lewis structure. 7 rows Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine.
Sulfur Dibromide Lewis Structure Sulfur Dioxide Chemical Compound Png 1024x476px Sulfur Dibromide Area Atom Black And
Some alternative names for this compound are sulfur monochloride the name implied by its empiricalformula SCl disulphur dichloride British English Spelling and sulphur monochloride British English Spelling.

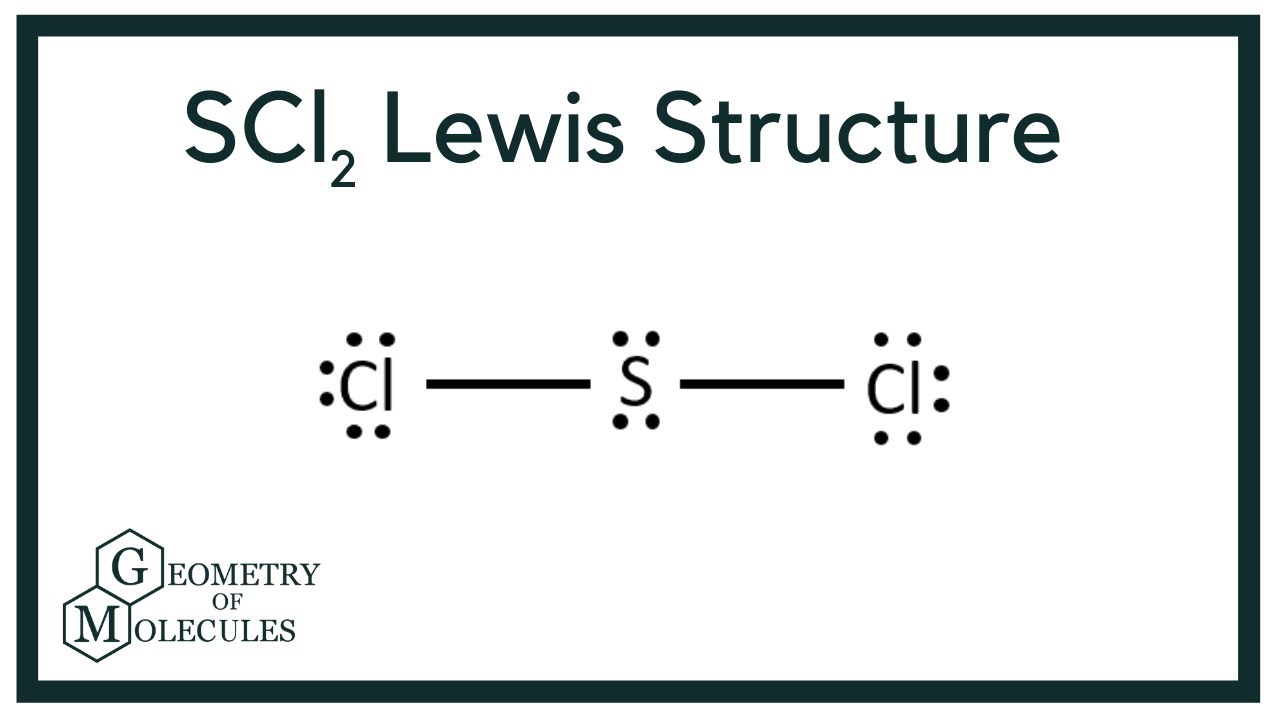
Lewis structure for sulfur dichloride. For the SCl2 Lewis structure use the periodic table to find the t. This Chlorine has 8 valence electrons as does this Chlorine. Number of nonbonding lone electron pairs around central atom.
Sulfur has 6 valence electrons. Sulfur and oxygen are in group 16 and bond similarly. Put two electrons between atoms to form a chemical bond.
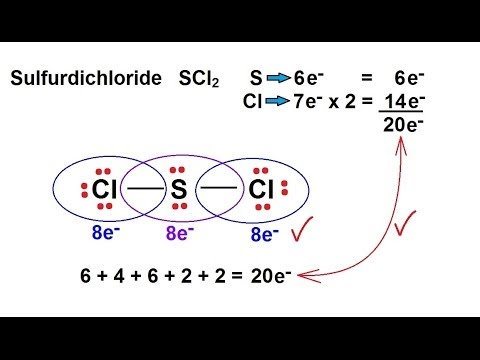
So 6 plus 28 is 34 total valence electrons. Average mass 102971 Da. Sulfur is the central atom surrounded by two chlorine atoms.
Follow some steps for drawing the lewis dot structure of ICl2-1. 8 rows SCl2 lewis structure contains one sulfur and two chlorine atom. XeO 2 F 2.
Central atom bond angles. Sulfur being the less. Has a similar shape to water due to 2 lone valence pairs on the sulfur.
So weve used all 20 of the valence electrons. Lets check and see if we have octets. Steps for Writing Lewis Structures.
Count total valence electron in ICl2-. These act as lone pairs and attach themselves on opposite ends of the Sulfur atom. Valence electrons used in Lewis structure.
Also the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell. Chlorine has 7 but there are four Chlorines. The valence electrons of Sulfur are 6 ie.
The fulfillment of the octet rule on each of the Chlorine atoms leaves 4 valence electrons. It is singly bonded to each chlorine atom. Put the least electronegative atom in the center.
SC12 6 Draw the Lewis structure. This is the SCl4 Lewis structure. Draw the Lewis-dot structure for sulfur dichloride SC12.
So they have octets. Sulfur chlorides carbon disulfide and carbon tetrachloride are removed from gases in 2 stages by sorption in disulfur dichloride with the temp of the first stage maintained at 10-30 deg and that of the second stage at -10 deg to -20 deg. Number of binding electron pairs around central atom.
H always goes outside. It has 6 electrons in its outermost shell and Chlorine has 7 electrons in its outermost shell. H 2 S NCl 3 OH -.
Total number of electron pairs around central atom. Sulfur is the least electronegative well put that at the center and then well put the Chlorines around the outside. Molecular Formula Cl 2 S.
Sulfur is the center atom. Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S 2 Cl 2. 70 More Lewis Dot Structures.
The sulfur atom has 4 lone pairs of electron while each chlorine atom has four lone pairs. A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride. Lets see how to draw the lewis dot structure of Iodine dichloride with easy steps.
Each of these atoms has an octet and were done. Lewis Dot of Sulfur Dichloride. This Sulfur atom forms two covalent bonds with each of the Chlorine atoms.
This cherry-red liquid is the simplest sulfur chloride and one of the most common. And then in the center Sulfur also has 8 valence electrons. If we talk about the chemical composition of the Sulfur dichloride the molecule consists of 2 atoms of chlorine and 1 sulfur atom.
Which statement best describes the correct Lewis-dot structure. Disulfur dichloride is the chemicalcompound of sulfur and chlorine with the formula S 2 Cl 2. Sulfur Dichloride comprises two Chlorine atoms separated by a lone Sulfur atom.
Monoisotopic mass 101909775 Da. Find the total valence electrons for the molecule. NOCl CF 2 Cl 2 HCN.
Thats the SCl2 Lewis structure. Some alternative names for this compound are sulfur monochloride the name implied by its empirical formula SCl disulphur dichloride British English Spelling and sulphur monochloride British English Spelling. Sulfur is the center atom that is double bonded to the chlorine atoms.

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

What Is The Name Of The Hybrid Orbitals Us Clutch Prep
Lewis Structure Sulfur Dioxide Resonance Sulfur Trioxide Png 1100x645px Lewis Structure Area Brand Carbon Dioxide Chemistry

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons
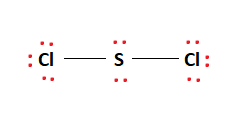
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Disulfur Dichloride Lewis Structure Sulfur Dichloride Text Logo Chemistry Png Pngwing

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules

Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Scl2 Lewis Structure Sulfur Dichloride Youtube

Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Drawings Dots Lewis

Hybridization Of Of2 Oxygen Difluoride In 2021 Molecules Oxygen Things To Come

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

How To Draw Lewis Structure For Scl2 Drawing Easy