N2o Lewis Structure Most Stable
One of the early questions asked by scientists once the concepts of atoms and molecules had been firmly established was how are atoms bonded. Hence the charge separation that is as that predicted by electronegativity.
The Most Stable Lewis Structure Of N2o Is
Indicate which of the structures you have drawn is the most likely structure and explain what is unfavorable with the others.
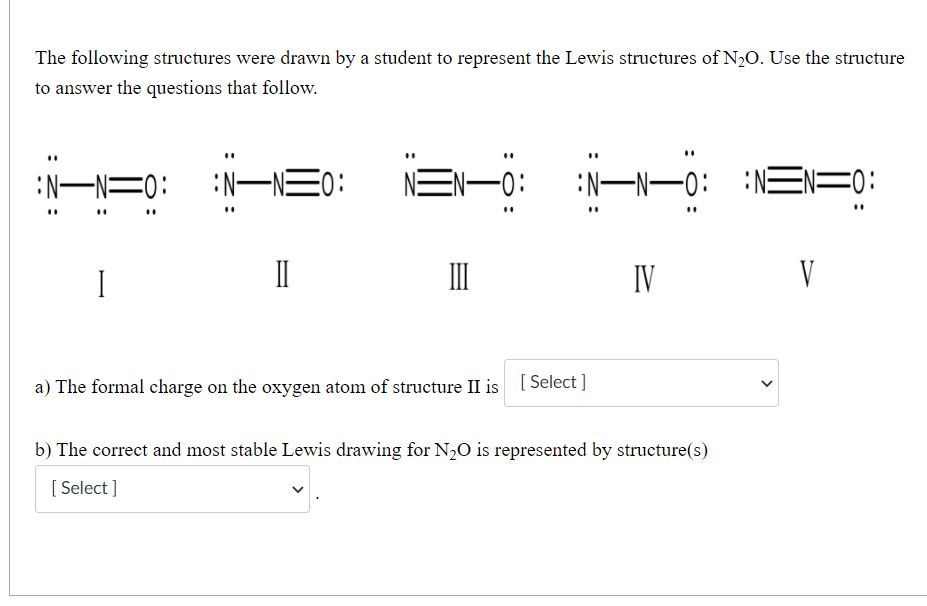
N2o lewis structure most stable. Resonance structures to identify the most stable structure of N2O. For the N2O Lewis structure there are a total of 16 valence electrons. A Lewis structure for NO would look like.
For the most stable molecule draw the 3-D molecular geometry and show the angles. Structure 3 is the best most stable structure we can draw for N 2 O. Are solved by group of students and teacher of NEET which is also the largest student community of NEET.
Remember that the most electronegative atom goes in the center of the structure. Dinitrogen trioxide Dinitrogen trioxide is a one of the oxides of nitrogens. Indicate the overall molecular dipole moment.
Try using these rules to create lewis structures for the following. The Lewis structure for N 2 O has three possible structures that all fill the octets of each atom. How many bonds does N2O.
There are three oxygen atoms around two nitrogen atoms. Now we are going to learn how to draw this lewis structure. Lastly there is a single unpaired electron on the nitrogen atom.
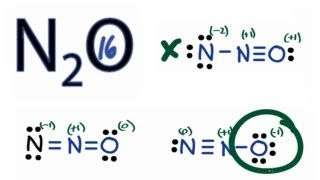
In this structure more electronegative O atom bears negative charge and less electronegative N atom bears a positive charge. These results in the most stable N2O Lewis structure. The most stable Lewis structure of N 2 O is represented by option D.
The double bar between the two chemical symbols means that nitrogen and oxygen share a double bond2 pairs of electrons. Note that a Lewis structure for carbon dioxide can be written using a carbon-oxygen single bond on one side and carbon-oxygen triple bond on the other. This chemistry video tutorial explains how to draw the lewis structure of n2o also known as nitrous.
N 2 O Formal Charge on each atom Show all numbers in each calculation. This makes sense because the Oxygen atom is more electronegative and therefore should have the negative formal charge. The structure with the -1 formal charge on oxygen would be more stable since oxygen is more electronegative than nitrogen and the structure is most stable with the negative formal charge on the most electronegative atom.
First we should try to draw the most stable lewis structure of N2O to decide the shape of N2O molecule. In this structure more electronegative. For N2O molecule.
A step-by-step explanation of how to draw the N2O Lewis Dot Structure Dinitrogen monoxide or Nitrous OxideFor the N2O structure use the periodic table to. The molecule SO3 has two coordinate bonds but that structure is not the most stable form as it carries a formal charge. Click to see full answer.
Nitrogen is the least electronegative in this molecule. Draw vectors showing polarity for all bonds. Calculate the Formal Charge FC on each atom in each structure and identify the structure that is most stable lowest in energy.
Draw three reasonable Lewis dot structures for eqN_2O eq. Is dative bond present in bcl3. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom.
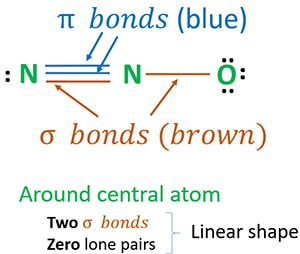
Its center atom contains around it Its center atom contains around it two sigma σ bonds. Lewis Structure of N2O. The formula N 2 O has five possible Lewis Structures as shown below.
Click hereto get an answer to your question the most stable lewis structure of n2o is. It also covers the molecul. This chemistry video tutorial explains how to draw the lewis structure of N2O also known as Nitrous Oxide or Dinitrogen Monoxide.
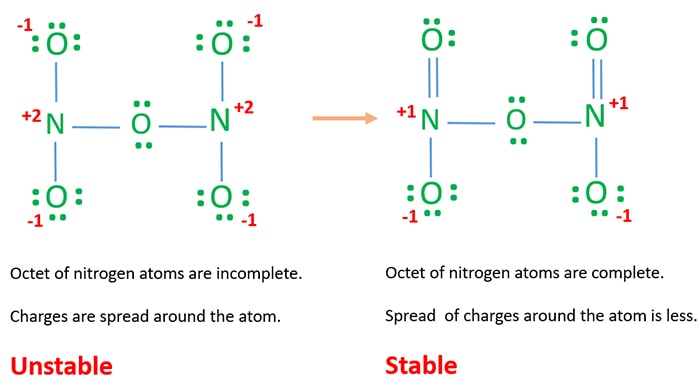
ADraw all possible Lewis structures assign the formal charges to all atoms and indicate the most stable Lewis structure. In Lewis Structure of N 2 O 3 one oxygen atom and nitrogen atom has -1 and 1 charges respectively. The observed bond lengths point to a bond order of 25 for the N-N bond and 15 for the N-O bond.
The rotationally resolved spectra of 15 N 15 N 16 O dimer for the polar and nonpolar isomers are studied in the region of the Nâ N stretching fundamental of the monomer â ¼2150 cm â 1 using a rapid-scan tunable diode laser spectrometer. The most stable Lewis structure of N2 O is represented by option D.

Nitrous Oxide N2o Has Three Possible Lew Clutch Prep
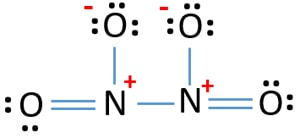
Lewis Structure Of N2o4 Dinitrogen Tetroxide Drawing Steps
N2o Lewis Structure Resonance Structures Oxidation Number
How Is The Lewis Structure For C3h6 Determined Quora

No Lewis Dot Structure Science Trends

N2o4 Dinitrogen Tetroxide Resonance Structures
N2o Lewis Structure Resonance Structures Oxidation Number
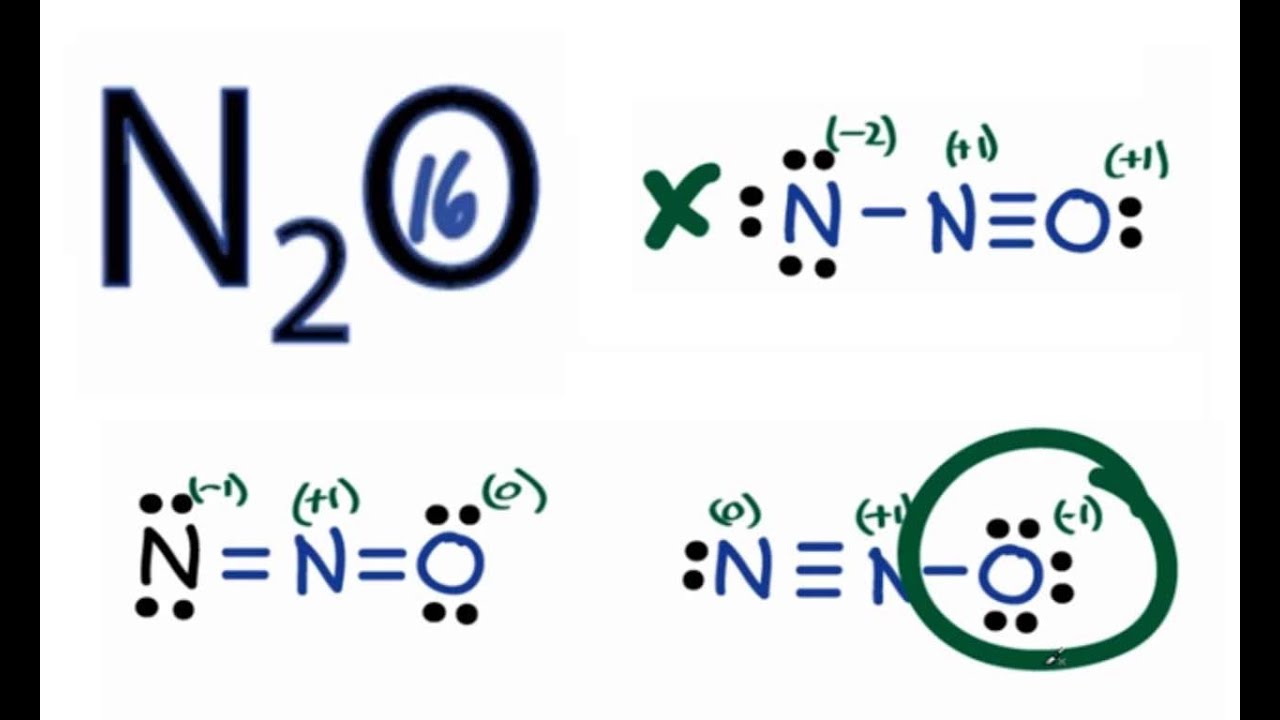
N2o Lewis Structure How To Draw The Lewis Structure For N2o Youtube
N2o5 Lewis Structure Resonance Structures Dinitrogen Pentoxide
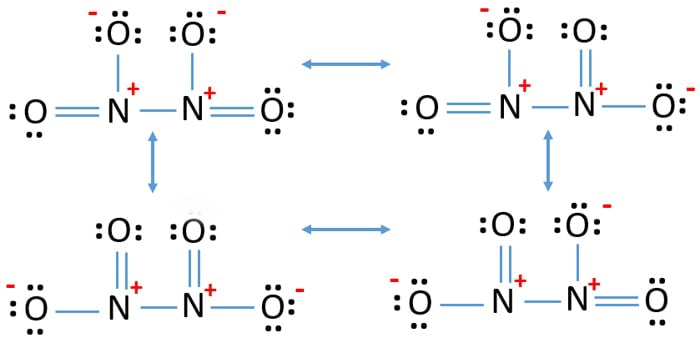
N2o4 Dinitrogen Tetroxide Resonance Structures
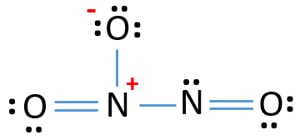
Lewis Structure Of N2o3 Dinitrogen Trioxide

N2o Lewis Structure How To Draw The Lewis Structure For N2o Youtube

The Most Stable Lewis Structure Of N2o Is
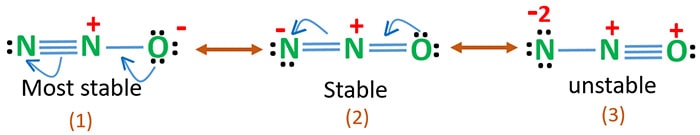
N2o Lewis Structure Resonance Structures Oxidation Number

Using Formal Charges To Evaluate Nonequivalent Resonance Structures Worked Example Video Khan Academy

N2o Lewis Structure Nitrous Oxide Youtube
The Following Structures Were Drawn By A Student To Chegg Com
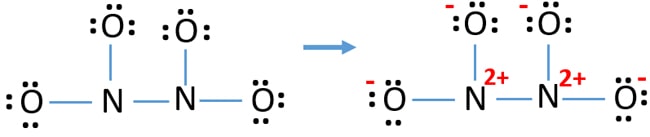
Lewis Structure Of N2o4 Dinitrogen Tetroxide Drawing Steps