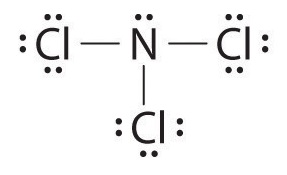
Nitrogen Trichloride Lewis Structure
Drawing the Lewis Structure for NCl 3. Images of the chemical structure of Nitrogen trichloride are given below.
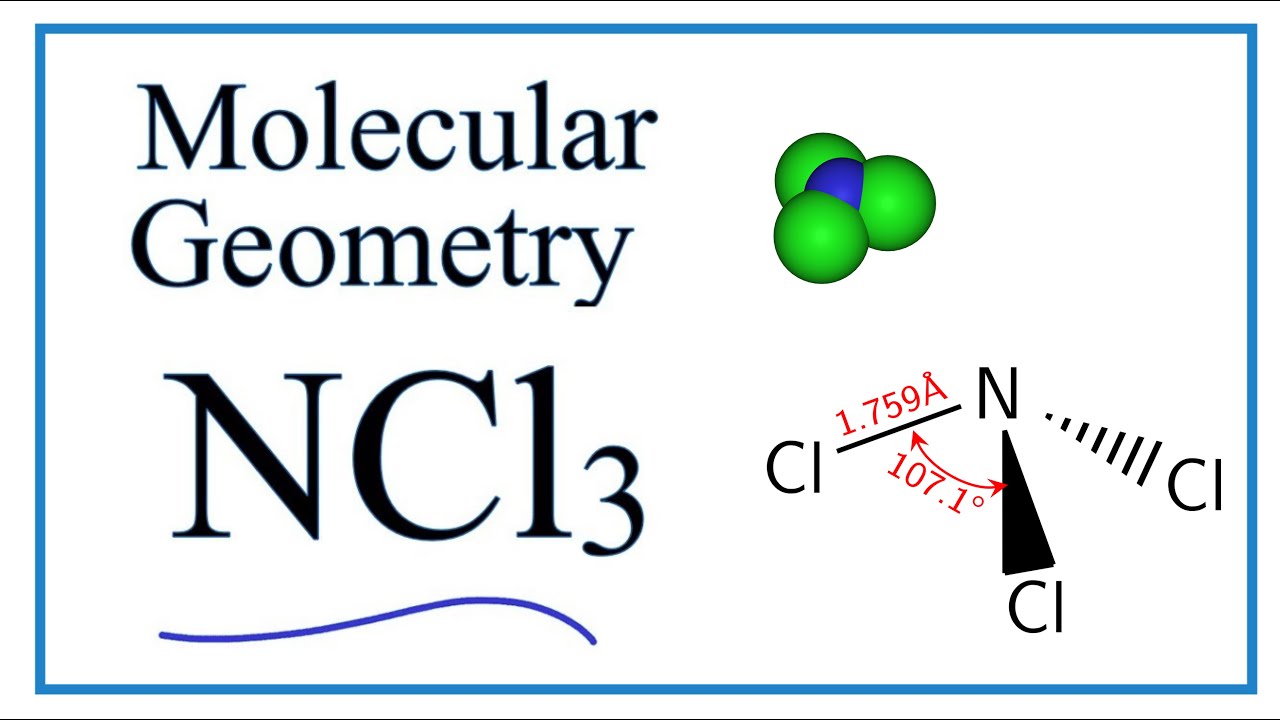
Ncl3 Molecular Geometry Shape And Bond Angles Youtube
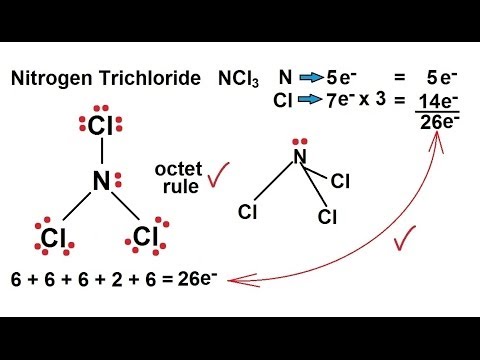
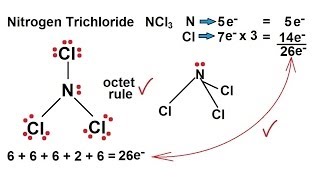
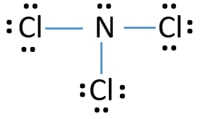
There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom.

Nitrogen trichloride lewis structure. Nitrogen trichloride also known as trichloramine is the chemical compound with the formula NCl3. Nitrogen trichloride is slightly polar in nature. In this video I will show the Lewis structure of nitrogen trichloride NCl3.
Presents dangerous fire hazard in the presence of reducing agents. NITROGEN TRIFLUORIDE is a very powerful oxidizing agent. Lewis structure of Nitrogen trichloride contains 1 lone pair and 3 bonded pairs.
This is mainly formed as a by-product when chlorine is treated with the ammonia derivative compounds. Also there are no charges on atoms in NCl3. Hydrolysis of nitrogen trichloride generates nitrogen gas and chlorine gas.
Nitrogen trichloride NCl3 lewis structure contains three N-Cl bonds. Emits toxic and corrosive fumes of fluoride when heated to decomposition Lewis 3rd ed 1993 p. On the other hand it is expected to be pyramidal according to the Valence-Shell-Electron-Pair-Repulsion VSEPR model of.
The Nitrogen trichloride molecule contains a total of 3 bond s There are 3 non-H bond s. Today in this video we will help you determine the Lewis Structure of NF3 molecule. This yellow oily pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine.
The total valence electron available for the NCl3 lewis dot structure is 26. If you can do those Lewis structures NCl 3 will be easy. It is considered to be a lachrymatory agent.
1 nitrate NO3 OR II nitrogen trichloride NCI3 A Draw the complete Lewis structure. When we consider both lone pairs and bond pairs we are referring to the Structure of the molecule. Trichloramine is another name for nitrogen trichloride.
This yellow oily pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reaction s between ammonia -derivatives and chlorine for example in swimming pool s. Nitrogen trichloride a simple molecule has long invited speculation about its unknown structure. Hence the Geometry of the molecule of NCl3 is Trigonal pyramidal.
2 B Identify the structure as being either polar nonpolar or ionic. 2 D State the hybridization of the central atom. The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral.
In the lewis structure of Nitrogen trifluoride NF 3 there are three N-F bonds and one lone pair on nitrogen atom. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. In the NH 3 Lewis structure Nitrogen N is the least electronegative so it goes in the center.
Each fluorine atom has three lone pairs. Three pairs will be used in the chemical bonds between the N and Cl and one pair of. The results showed both chemicals to have an irritant potency of the same order of magnitude.
On the one hand it is isoelectronic with NSiH a planar molecule. NF3 Lewis Structure Nitrogen Trifluoride Were you searching for a short yet detailed video on NF3 Lewis Structure. A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together.
Learn this topic by watching Lewis Dot Structures. Nitrogen trichloride is a yellow oily liquid with its pungent odor. In the Lewis Dot Structure of nitrogen trichloride NCl 3 how many shared valence electrons are around the central atom.
1 C Name and draw the VSEPR shape for this structure. In the Lewis structure for NCl 3 there are a total of 26 valence electrons. It consists of one Nitrogen and three Fluorine atoms.
Nitrogen trichloride also known as trichloramine is the chemical compound with the formula NCl 3. All Chemistry Practice Problems Lewis. Chlorine and nitrogen trichloride showed dissimilar concentration-response curves.
Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Alongside monochloramine and dichloramine trichloramine is responsible for the distinctive chlorine smell associated with swimming. The hybridization of NCl3 is Sp³.
NCl 3 is similar to NH 3 and NF 3. If yes then we have got you. Hence the Structure of the molecule of NCl3 is Tetrahedral since a lone pair of electron can be considered to be a bond pair.
While the maximal response of nitrogen trichloride was reached in 10 min the maximal response of chlorine was reached between 45 and 60 min of exposure. Etches glass in the presence of moisture. The molecular mass of Nitrogen trichloride is calculated as below Mol mass of NCl3 1 14 mol mass of N 3 3545 mol mass of Cl 120365 gmol.
Neutral Compounds Concept Videos. Each step of drawing the lewis structure of NF 3 is.
Is Ncl3 Polar Or Non Polar Quora
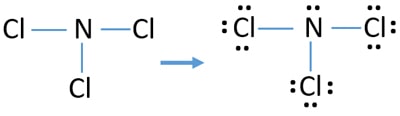
Ncl3 Nitrogen Trichloride Lewis Structure

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Draw The Lewis Dot Structure For Ncl3 Clutch Prep

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Is Ncl3 Polar Or Nonpolar Explain Study Com
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube
Ncl3 Lewis Structure Studyrankersonline
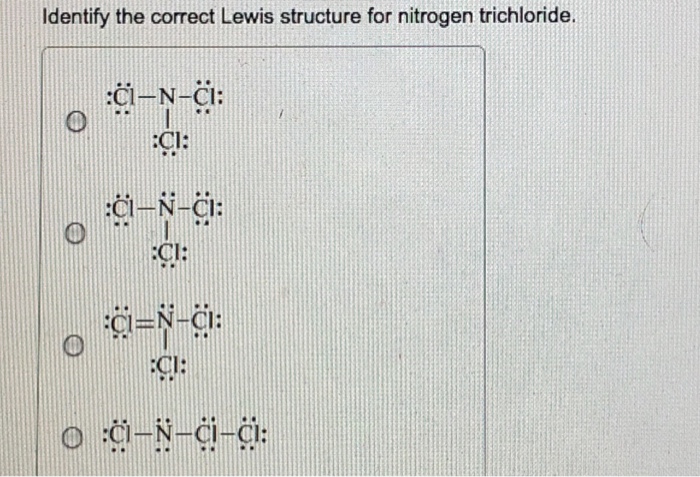
Identify The Correct Lewis Structure For Nitrogen Chegg Com

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Nitrogen Trichloride Alchetron The Free Social Encyclopedia

The Correct Dot Formulation For Nitrogen Trichloride Has

Determine The Electron Geometry Eg And M Clutch Prep
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Lewis Structure Of Ncl3 Nitrogen Trichlorode Youtube
Ncl3 Nitrogen Trichloride Lewis Structure

Choose A Lewis Structure For Ncl3 Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube