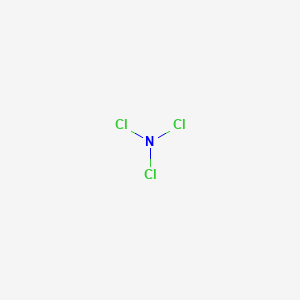
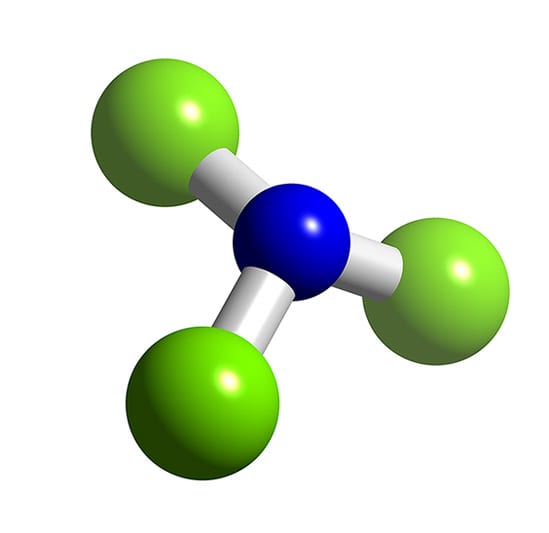
Nitrogen Trichloride Synthesis
Nitrogen trichloride is an unstable compound which can be formed during the electrolytic production of chlorine from salt solutions which contain ammoniacal compounds. Five minutes later the mixture exploded violently.

Less Energetic Basic Compounds Energeticchemical
Simon Cotton University of Birmingham.

Nitrogen trichloride synthesis. A Synthesis of the Toxic Factor from Methionine Bentley H. Action of nitrogen trichloride on proteins. Nitrogen Trichloride Chemicals-Ammonium Nitrate NH4NO3-Chlorine Cl-Water H20HOH Procedure.
Action of nitrogen trichloride on certain proteins. In rabbits S-methylcysteine sulphoximine does not produce the toxic symp-toms obtained with methionine sulphoximine. DIMETHYLSULPHOXIDE is converted by means of the Schmidt reaction 1 to a product identified as I R -CH 3 by.
Action of Nitrogen Trichloride on Proteins. During preparation of nitrogen trichloride by bubbling chlorine into an aqueous solution of ammonium sulfate and 100 mL of the ether 50 mL of the ether-NCl3 mixture over ammonium sulfate solution was stored in a refrigerator. ClCl 580 kcal mol 1 and has a low basicity toward boron atoms and thus favors radical pathways.
101038165735b0 No abstract available. This reagent is characterized by a weak NCl bond NCl 477 kcal mol 1. Broadly the invention involves the preparation of nitrogen trichloride by the reaction of chlorine on a solid ammonium salt in the presence of sufiicient moisture to permit the reaction to.
S-methylcysteine sulphoximine I R -CH2CHNH2COOH R CH and dimethyl-sulphoximine I R R CH are prepared by a similar method from the corresponding sulphoxides. Gently heat the flask while bubbling the gas through. Use a Chlorine bubbler and bubble the chlorine gas through the Ammonium NitrateWater solution.
The free-radical nature of this process has been proven by Brown using radical clock experiments. Nitrogen trichloride synthesis. Trichloroamine NCl 3 The explosive liquid that injured both Sir Humphrey Davy and Michael Faraday.
Authors H R BENTLEY E E McDERMOTT J K WHITEHEAD. The analysis is performed by a laser Raman spectrometer. Proc R Soc Lond B Biol Sci.
In a 200mL flask take 30g Ammonium Nitrate and add it to 70mL of Water. This turned out to be much less reactive than nitrogen trichloride. MISANI F REINER L.
It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride nitrogen tribromide and nitrogen triiodide all of which are explosive. Molecule of the Month April 2017 Also available. After first attempting the synthesis in 1903 Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride.
Nitrogen trichloride a moderately difficult-to-handle reagent reacts with trialkylboranes to give one equivalent of the alkyl chloride. Alone among the nitrogen trihalides it has a negative enthalpy of formation. Studies on nitrogen trichloride-treated prolamines.
Synthesis of methionine sulphoximine and other sulphoximines. Nitrogen trichloride decomposes spontaneously and explosively at various concentrations depending on the temperature but usually at concentrations of 3 ww. After the first attempt at synthesis in 1903 Otto Ruff produced 25 years later nitrogen trifluoride by electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride.
Synthesis of the toxic factor. There is disclosed an improved process for the preparation of phosphorus trichloride wherein elemental phosphorus is caused to react with chlorine. A synthesis of the toxic factor from methionine Nature.
The improvement comprises continuous high speed analysis of the contents of the reactor whereby the reaction is controlled to produce less phosphorus pentachloride and other by-products.

Nitrogen Trichloride An Overview Sciencedirect Topics

Webelements Periodic Table Nitrogen Nitrogen Trichloride

How To Balance Cl2 N2 Ncl3 Chlorine Gas Nitrogen Gas Youtube

How To Balance N2 F2 Nf3 Nitrogen Gas Fluorine Gas Youtube
Ncl3 Molecule Of The Month April 2017 Html Only Version

Action Of Nitrogen Trichloride On Proteins A Synthesis Of The Toxic Factor From Methionine Nature
Ncl3 Molecule Of The Month April 2017 Html Only Version

Explosion Phenomena Of Nitrogen Trichloride Taken With High Speed Download Scientific Diagram

Nitrogen Trichloride An Overview Sciencedirect Topics
Ncl3 Molecule Of The Month April 2017 Html Only Version

Nitrogen Chloride Ncl3 6ci 7ci 8ci 9ci 10025 85 1 Wiki
Ncl3 Molecule Of The Month April 2017 Html Only Version
Nitrogen Trichloride Ncl3 Pubchem

Nitrogen Trichloride An Overview Sciencedirect Topics
Nitrogen Trichloride Sciencemadness Wiki

Nitrogen Trichloride An Overview Sciencedirect Topics

Webelements Periodic Table Nitrogen Nitrogen Trifluoride