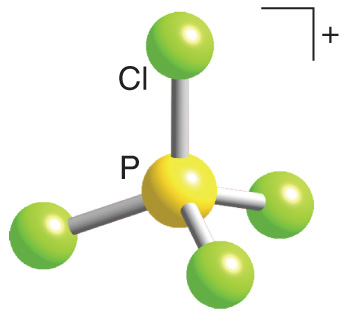
Pcl4- Molecular Geometry
For each molecule on the worksheet the lewis dot structure the number of valence electrons the electron arrangement ea and the molecular geometry mg o3. PCl4 cation is a AX4 type ion.

The Shape Of Covalent Molecules Ppt Video Online Download
By signing up youll get thousands of step-by-step solutions.

Pcl4- molecular geometry. CCl4 is also called carbon tetrachloride. The geometry of XeOF2 is consistent with a trigonal bipyramidal AX2YE2 VSEPR arrangement that gives rise to a T-shaped geometry in which the two free valence electron lone pairs and Xe-O bond domain occupy the trigonal plane and the Xe-F bond. Valence electrons of P 5.
Yea it becomes easy to understand the molecular geometry around a central atom BF4- c. What is the the shape molecular geometry of PCl4. Have no lone pairs in any case so the angles are ideal.
Gaseous beryllium hydride BeH 2 Q H X Be. 1031 a A which has a square planar molecular geometry has the most electron. P and Chlorine Atomic number.
What is the bond angle of pcl4. Nonpolar compounds either have no polar bonds or contain symmetrical polar bonds. 5 73.
Predict the electron pair geometry and the molecular structure of PCl4-. Now we will calculate the number of. CH4 CCl4 PCl4 PCl6-SF6 H3NBF3 NH3BF3 All is described and explained.
Polar molecules must contain polar bonds due to a difference in electronegativity between the atoms. Quiz your students on PCl4 Lewis Dot Structure - Bond Angle Hybridization Molecular Geometry using our fun classroom quiz game Quizalize and personalize your teaching. It has four bonding pairs with no lone pairs.
Four electron pairs are distributed in a tetrahedral shape. The phosphorous atom in PCl4 is sp3 hybridized and the geometry is tetrahedral. Lets do the lewis structure for ccl4 carbon tetrachloride sometimes just called carbon tet.
The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry. The shapes of P C l4. What is PCl4 in chemistry.
What is the shape of XeOF2. The shapes of PCl4 PCl4 and AsCl5 are tetrahedral see-saw and trigonal bipyramidal respectively. Four pairs will be used in the chemical bonds between the P and F.
Monovalent side atoms 4. Made with Explain Everything. Total number of valence electrons of PCl3.
PCl 4-is a negative ion an anion so you have to an. A Phosphorus Pentachloride molecule consists of 1 atom of phosphorus for 5 atoms of chlorine. What shape is pcl6.
Understanding the molecular structure of a compound can help determine the polarity reactivity phase of matter color magnetism as well as the biological activity. Tetrahedral see-saw and trigonal bipyramidal. How many electrons are in the expanded Valence in xeof2.
Thus we see that BCl 3 contains three bonds and there are no lone pairs of electrons on boron. Pcl6- molecular geometry E-Mail. The Molecular Geometry shape Of The PF4 Ion IS Tetrahedral Trigonal Planar Trigonal Bipyramidal Octahedral Trigonal Pyramidal Using VSEPR Theory The Predicted Cl-B-Cl Bond Angle For BCI3 Is 180 107 120 1095 900 Using VSEPR Theory The Molecular Geometry shape For SF4 Is Square Planar Trigonal.
The dipole moment of a polar molecule is always equaled to non zero and nonpolar molecules always have zero dipole moment. Molecules having a molecular formula of ax4e have trigonal bipyramidal molecular geometry. Pcl3 is a lewis base so.
Valence electrons of Phosphorus Valence electrons of Chlorine. Pcl4 sp3 tetrahedral shape. B We write the Lewis structure of BCl 3 as.
Overall charge on P Cl 4 P C l 4 1. If we talk about the physical appearance of the compound it is sensitive to water and. Are tetrahedral see-saw and trigonal bipyramidal respectively.
10 Pcl4 Lewis Structure. P Cl 4 P C l 4. Compared to what might be expected from the simple geometry of the shape.
Phosphorus Pentachloride or PCl5 is a compound formed by chemical elements Phosphorus Atomic number. What is the the shape molecular geometry of PCl4. Po33- pcl4 molecular geometry the Lewis structure for PCl 4-there are a total of 34 valence electrons around a atom.
Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3. When copper is heated with an excess of sulfur copperi sulfide is formed.

What Is The Molecular Geometry Shape Of Sulfur Tetrafluori Clutch Prep
Http Www Rapidlearningcenter Com Solutions Ap Chemistry Drills24 Apc Ps13 Lewisstructures Pdf

Pcl6 Lewis Structure How To Draw The Lewis Structure For Pcl6 Youtube

The Shape Of Covalent Molecules Ppt Video Online Download

Pcl4 Lewis Structure How To Draw The Dot Structure For Pcl4

How To Draw The Lewis Structure For Pcl4 Youtube

The Shapes Of Pcl 4 Pcl 4 And Ascl5 Are Respectively

Drawing The Lewis Structure For Pcl4 Youtube
Https Nonsibihighschool Org Advancedch11quiz1answers Pdf
Https Nonsibihighschool Org Advancedch11quiz1answers Pdf

Drawing The Lewis Structure For Pcl4 Youtube

The Shapes Pcl4 Pcl4 And Ascl5 Are Respectively
The Shapes Pcl4 Pcl4 And Ascl5 Are Respectively A Square Class 11 Chemistry Cbse