Pf5 Lewis Structure Resonance
B Is the PF3 molecule polar or is it nonpolar. B 1s 2 2s 2 2p 6 3s 2.
Https Www Ionicviper Org System Files Understanding 20hypervalency Pdf
A H 2 O.
Pf5 lewis structure resonance. But check the formal charges -- its not the best Lewis. Well put the Phosphorus in the center and then the Fluorines we have five of them lets put them around it like this. D 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p5.
In each case explain your prediction. Fluorine group 7 but we have five of those so we need to multiply that 7 by 5. Some molecules are not well defined by a single lewis structure.
Sufur trioxide is an example. 1-7 Draw the Lewis structures for PF 3 and PF 5. Some hints are given.
1-6 Name the element that corresponds to each electronic configuration and identify how many valence electrons it has. On the basis of structural and bonding considerations account for the fact that NF3 and PF5 are stable molecules but NF5 does not exist. Draw the Lewis dot structures and resonance structures for the following.
For such a molecule several dot structures may be drawn. Determine the central atom in this molecule. I NF5 ii.
A Draw the Lewis electron-dot structures for PF3 and PF5 and predict the molecular geometry of each. For these structures experimental data tells us a single Lewis structure isnt an accurate picture of the molecule as found in the real world. Thus PF5 has net four covalent bonds and one ionic bond.
Write Lewis structures that obey the octet rule for each of the following. How many total valence electrons are there in the molecule. Show ALL resonance structures where.
The more stable structures contribute more than less stable ones. We are being asked to identify the resonance forms of S 3. This one is a bit tough since the first Lewis structure you generate will seem like the right one.
Bis the PF3 molecule polar or is it nonpolar. For the molecule phosphorous pentafluoride PF5. A Draw the Lewis electron-dot structures for CO32- CO2 and CO including resonance structures where appropriate.
In this picture the dashed line represents the ionic bond F P F F F-F FP F-F F F P F F-F F P F F F F--F P F F F F. 3 a CHCl3 b NH4 c H2CO 6. C On the basis of bonding principles predict whether each of the following compounds exists.
For resonance structures the skeleton of the molecule or ion stays in the same. Does phosphorous pentafluoride have any resonance structures yes. A single bond in a Lewis structure represents 2 electrons.
All the dot structures contribute to the real structure. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. Explain C on the basis of bonding principles predict whether each of the following compounds exists.
A double-headed arrow between Lewis structures indicates that they are resonance forms. Lewis structures resonance forms. For many structures O 3 NO 3- etc drawing a single structure wont show the actual distribution of electrons between atoms.
C 1s 2 2s 2 2p 4. NF3 and PF5 are stable molecules. Thus the electronic structure of the NO 2 ion is shown as.
Lewis structures have a major flaw. 1-5 Draw the Lewis structures for the following compounds. The Lewis structures of some molecules do not agree with the observed structures.
Consider the molecules PF3 and PF5. On the periodic table Phosphorus is in group 5 it has 5 valence electrons. Write Lewis structures for the following molecules or ions which have central atoms that do not obey.
A 1s 2 2s 2 2p 6. The actual electronic structure of the molecule the average of the resonance forms is called a resonance hybrid of the individual resonance forms. What is the steric number.
The actual distribution of electrons the resonance hybrid is an average of the distribution indicated by the individual Lewis structures the resonance. As you draw them keep in mind that some of the resonance structures may not satisfy the octet rule. Lets do the Lewis structure for PF5.
First we will have to draw the Lewis Structure of S 3. For example the NO2 molecule has an odd number of electrons thus the octet rule cannot be satisfied for the nitrogen atom. Calculate the total number of valence electrons present.
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. Consider the molecules PF3 and PF5. Write the Lewis electron-dot formulas for these molecules.
There is only 1 atom in the structure Sulfur is the central atom. To get around this we draw resonance. PF5 SF6 elements from the third period on have unfilled d orbitals that can acomodate additional electrons.
Case they do not exceed the octet rule. With this model we can draw a series of resonance structures as shown below for PF5. A On your work draw the Lewis structure of phosphorous pentafluoride and label the formal charge of any atom with a non-zero formal charge.
Https Pubs Acs Org Doi Pdf 10 1021 Acs Jchemed 0c00368
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
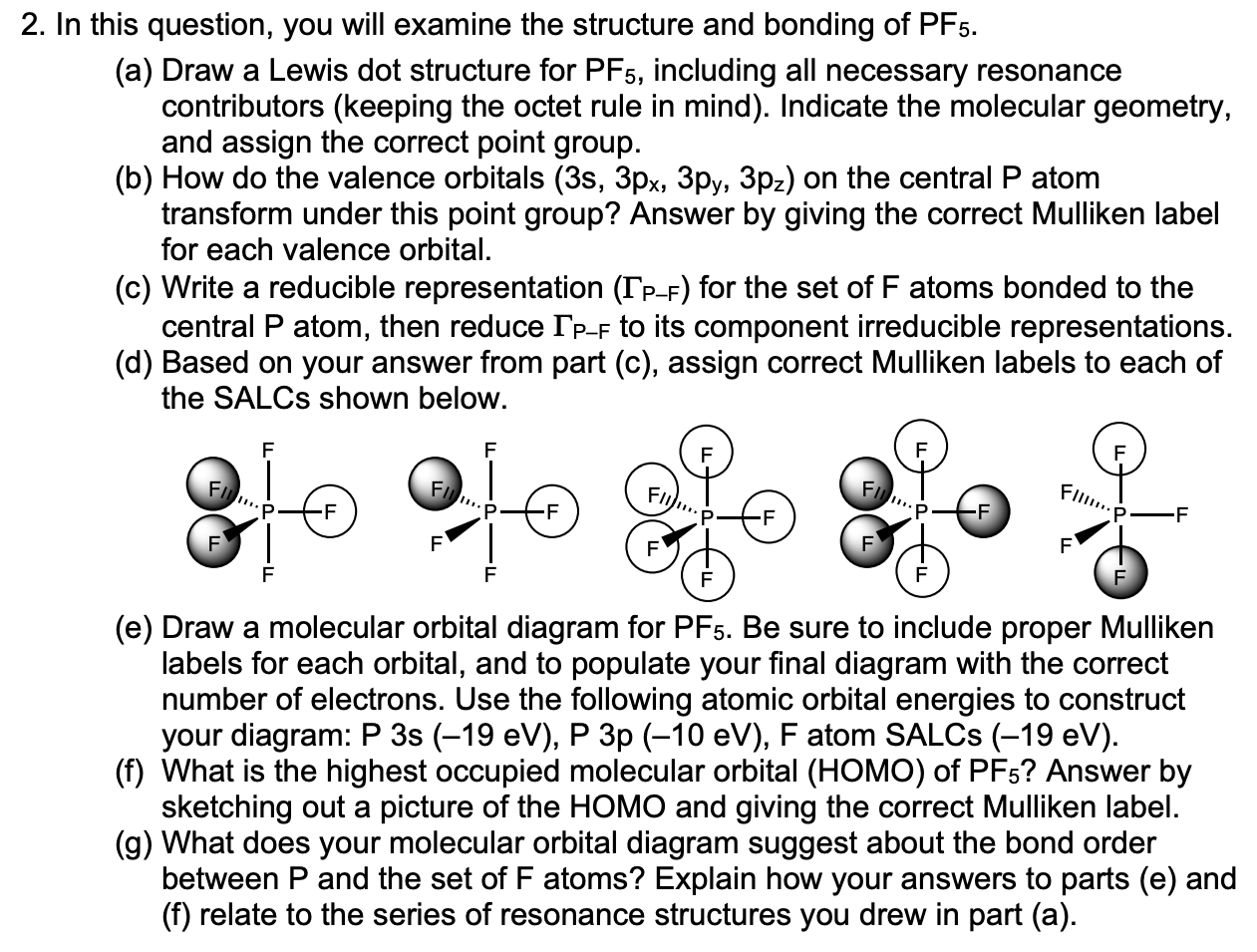
2 In This Question You Will Examine The Structure Chegg Com

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
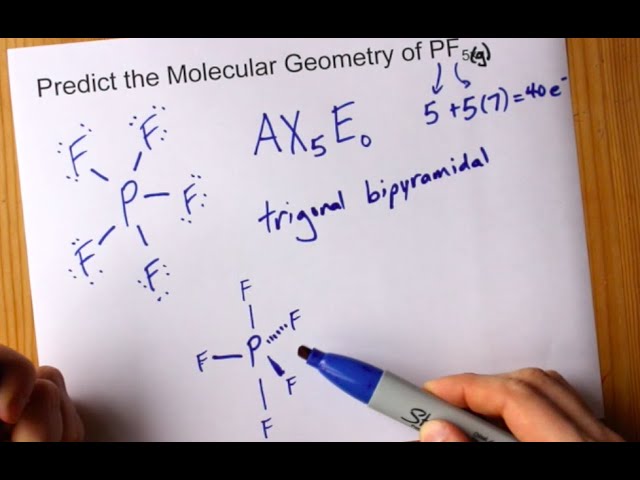
Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
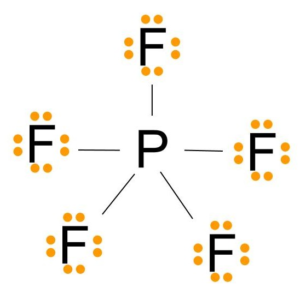
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
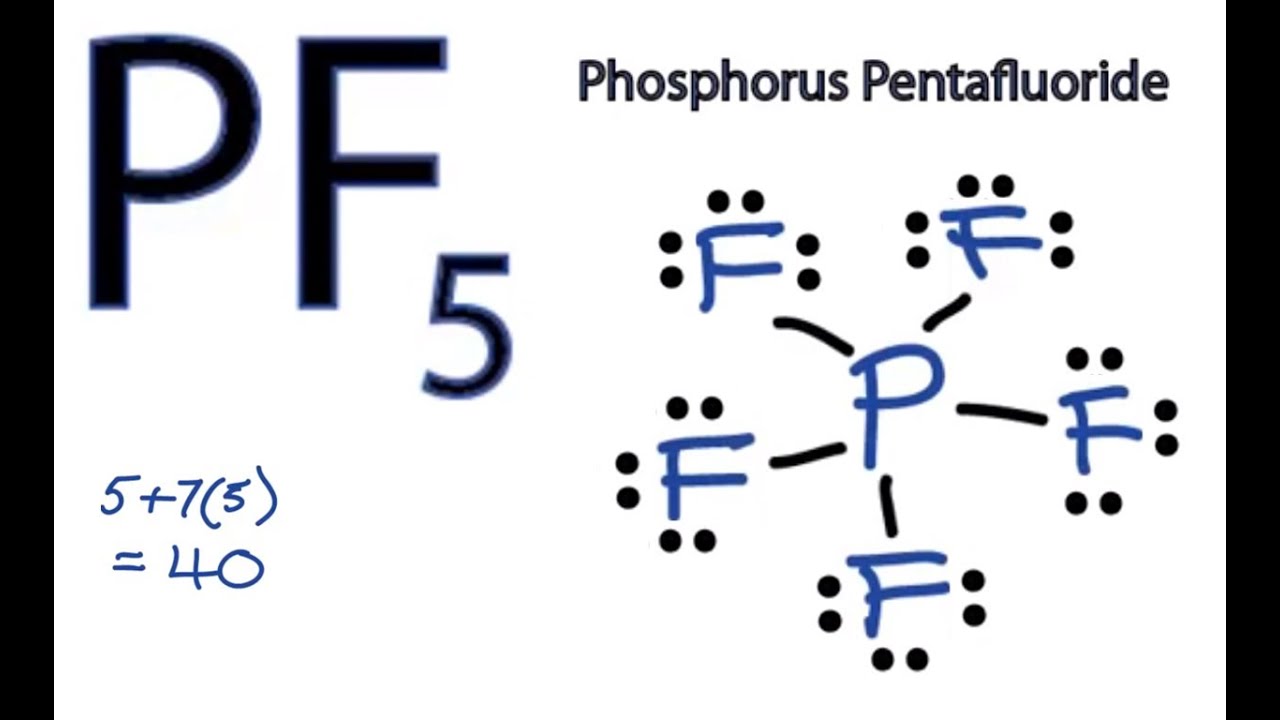
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
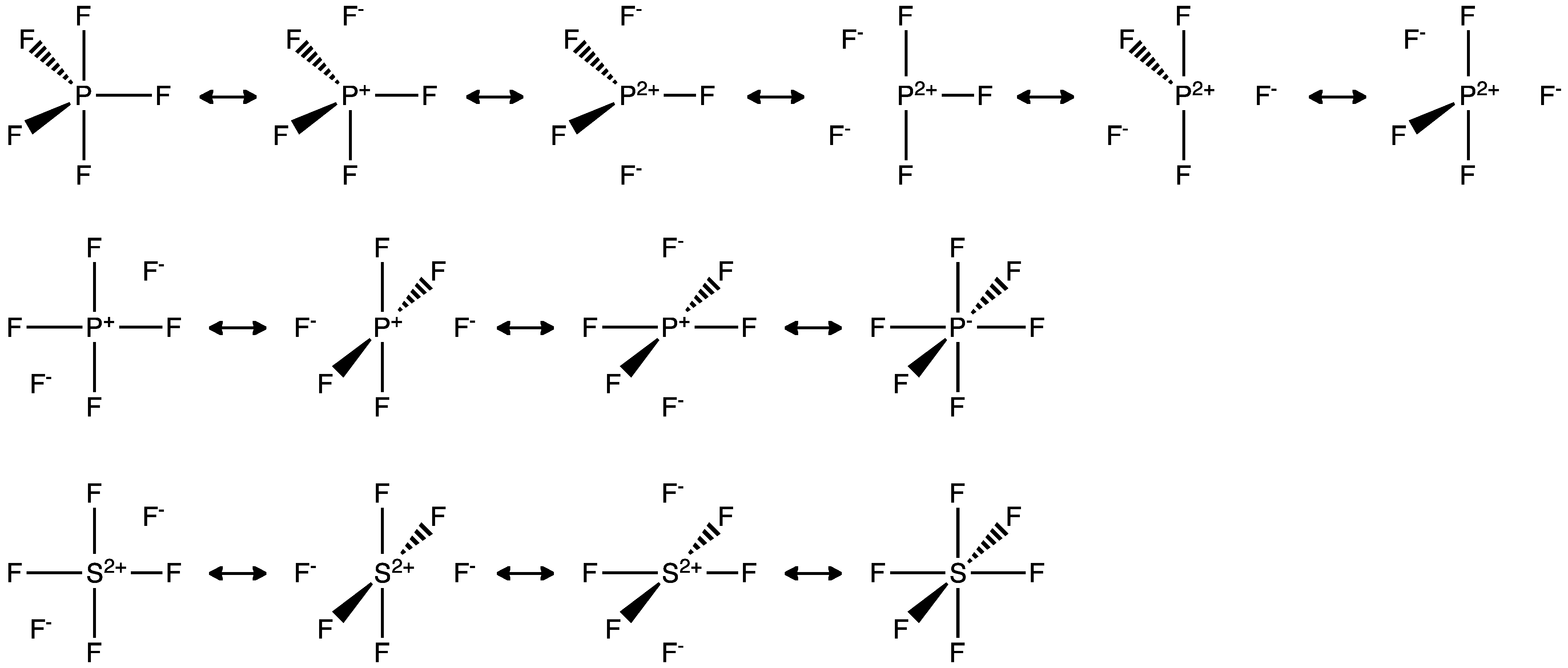
File Pf5 Sf6 Pf6 Hypervalent Structure Resonance Png Wikimedia Commons
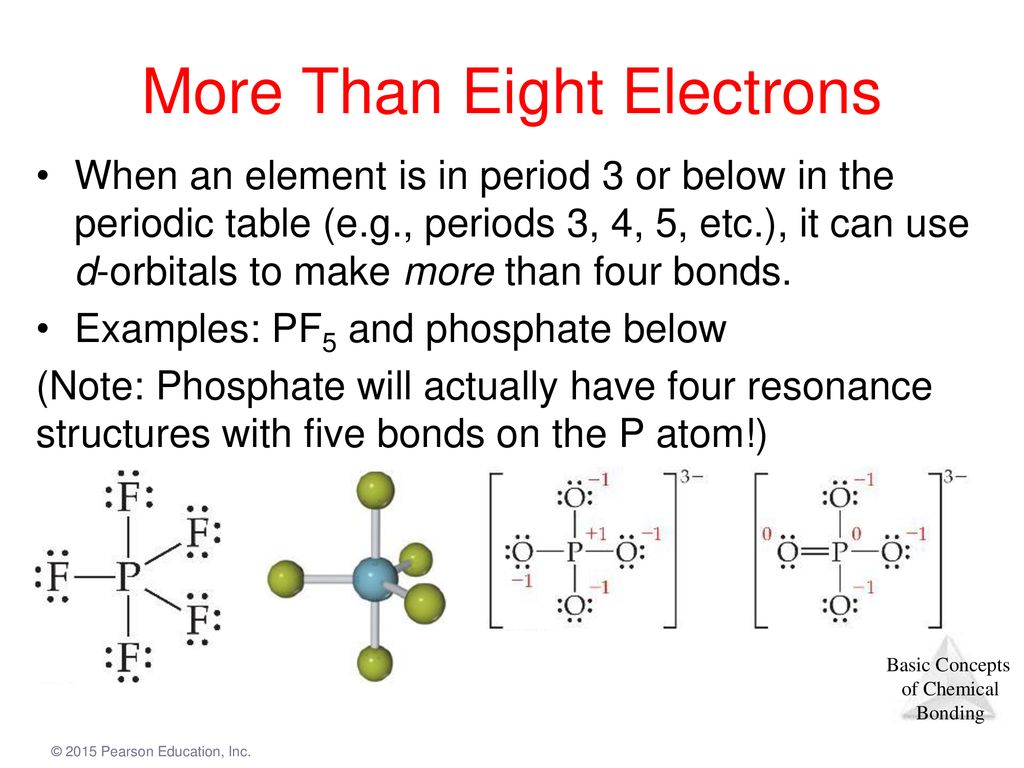
Lecture Presentation Unit 6 Chemical Bonding Day 2 Formal Charge Resonance Structures And Bond Enthalpy Ppt Download

Pf5 Molecular Geometry Shape And Bond Angles Youtube

Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Pf5 Lewis Structure Lewis Structure Of Pf5 Phosphorus Pentafluoride Draw Lewis Structure For Pf5 Youtube
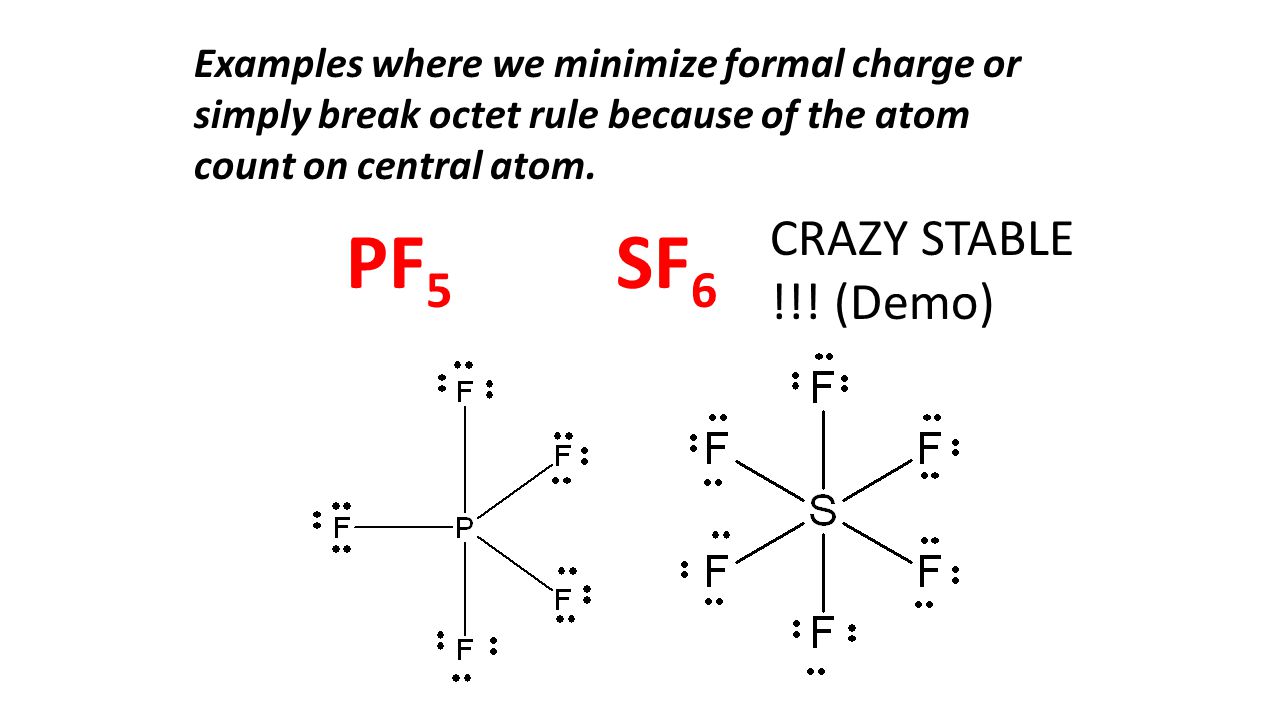
Octet Rule Structures Ppt Video Online Download
