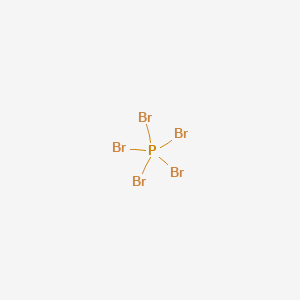Phosphorus Pentabromide Lewis Structure
ReadDownload File Report Abuse. The molecular structures of PH3 and NH3 are the same.

Pbr5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
When heated to decomposition it emits toxic fumes of bromide and oxides of phosphorus Lewis 3rd ed 1993 p.

Phosphorus pentabromide lewis structure. PBr5 Lewis StructureLewis Structure of PBr5 Phosphorus PentabromideDraw Lewis Structure for PBr5. Gaseous and molten PCl 5 is a neutral molecule with trigonal bipyramidal geometry and D3h symmetry. The structure of PCl 5 depends on its environment.
Phosphorus Pentabromide Lewis Dot Structure pbr5 lewis structure geometry molecular phosphorus shape bond pentabromide pbr bromine valence electrons angle study hybridization seven five each lewis phosphorus structure trichloride pentachloride electron pentafluoride molecular outros estrutura atom iodo favpng pngwing bromoform phosphorus pentabromide svg commons formula. In the Lewis structure for PBr 5 there are a total of 40 valence electrons. Lewis Structure - Phosphorus Pentabromide Tribromide Crystal - Ytterbiumiii Bromide Free PNG is a 1100x880 PNG image with a transparent background.
Phosphorus has five valence electrons and each bromine has seven valence. It appears to be a yellow crystalline solid. PHOSPHORUS PENTABROMIDE is a yellow crystalline compound toxic and corrosive.
Posted on October 21 2016. Phosphorpentabromid ist ein rotgelber Feststoff mit stechendem Geruch dessen Kristalle aus PBr 4 Br -Ionen bestehen. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide PBr 4 Br 3.
Assignment 7 and Practice Third Exam Solutions a Why is chlorine pentafluoride polar but phosphorus pentafluoride isnt. We have 5 plus 7 times 5 is 35 so we have 40 total valence electrons. The Lewis structure of PBr 5 or phosphorus pentabromide is.
PBr5 Lewis Structure Molecular Geometry Hybridization and Polarity. Oberhalb von 35 C zersetzt sich die Verbindung zu Phosphor III-bromid und Brom. Hi this is Dr.
Contact with water or steam leads to violent decomposition hydrolysis producing toxic and corrosive phosphoryl bromide fumes. It is comprised of one atom of phosphorus P bonded to three hydrogens H. A step-by-step explanation of how to draw the PBr5 Lewis Dot Structure Phosphorus PentabromideFor the PBr5 structure use the periodic table to find the to.
In the solid-state the compound exists as PBr4 Br- and in the gaseous state it dissociates. Phosphorus pentabromide written as PBr5 in the chemistry equations is a reactive yellow solid. Halogens are highly reactive and electronegative molecules.
Become a member and unlock all. Notable Exceptions to the Octet Rule. The structures for the phosphorus chlorides are invariably consistent with VSEPR theory.
Er ist extrem hygroskopisch hitze- und wärmeempfindlich und wirkt korrodierend. PBr5 or Phosphorous Pentabromide is a compound that consists of 5 molecules of Bromine and 1 molecule of Phosphorus. Tagged under Lewis Structure Phosphorus Tribromide Solid Phosphorus Phosphorus Heptabromide.
Put the Phosphorus in the center and then the Brs go on the outside like so. Phosphorus pentabromide is a reactive yellow solid of formula P Br 5 which has the structure PBr 4 Br in the solid state but in the vapor phase is completely dissociated to PBr 3 and Br 2. The Lewis structure of PBr 5 or phosphorus pentabromide is.
In the Lewis structure for PBr 5 there are a total of 40 valence electrons. Phosphorus trihydride or phosphine has the formula PH3. Bromine is a halogen from Group 17 of the periodic table.
It is a chemical formula for Phosphorus Pentabromide. It appears to be a yellow crystalline solid. If you can do those Lewis structures PBr 3 will be easy.
Feb 15 2021 - In this video we are going to learn about the Lewis structure of PBr5. Še eo - s 3 eyss. Phosphorus has 5 valence electrons plus 7 for Bromine but we have five Bromines.
Phosphorus Pentabromide Lewis Structure Free PDF eBooks. Five pairs will be used in the chemical bonds between the P and Br. Lets do the Lewis structure for PBr5.
A step-by-step explanation of how to draw the PBr5 Lewis Structure Phosphorus Pentabromide. The compound has one molecule of Phosphorus and five Bromine molecules. The structure of PBr5 in the solid-state is PBr4 Br whereas in the vapor phase it dissociates to become PBr3Br2.
In the PBr 5 Lewis structure Phosphorus P is the least electronegative so it goes in the center.
Pbr5 Molecular Geometry Lewis Structure Shape Bond Angle And More

Phosphorus Pentabromide Nitrogen Tribromide Phosphorus Tribromide Tetrabromomethane Angle White Png Pngegg

Phosphorus Pentachloride Png Images Pngwing
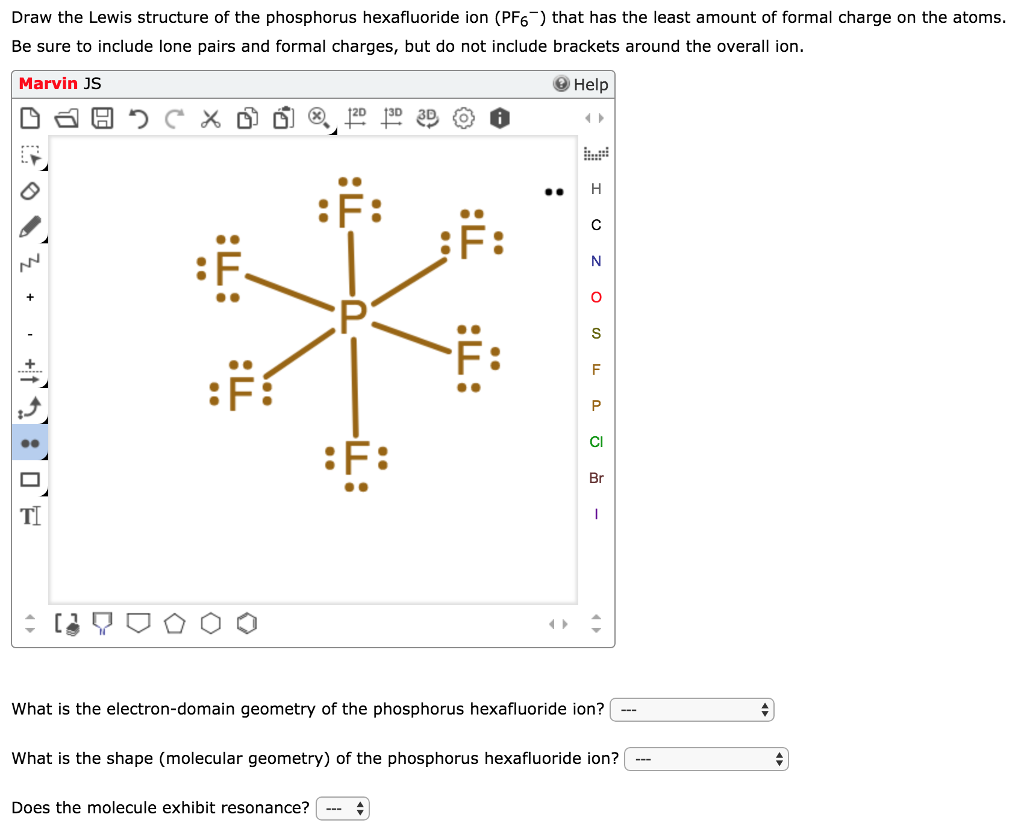
Draw The Lewis Structure Of The Phosphorus Chegg Com

Pbr5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Occurrence Preparation And Properties Of Phosphorus

Pbr5 Lewis Structure Phosphorus Pentabromide
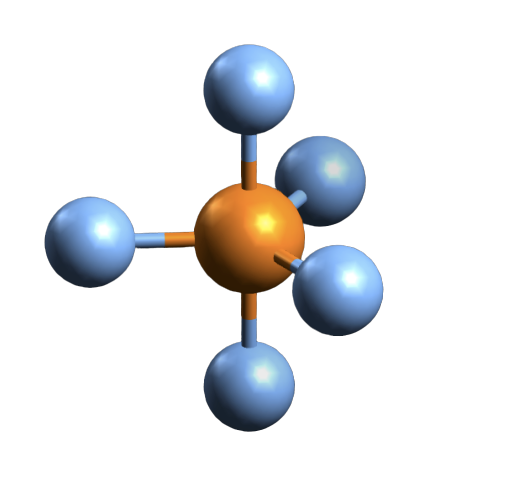
Pbr5 Molecular Geometry Lewis Structure Shape Bond Angle And More

Oneclass What Is The Lewis Structure For Phosphorus Pentabromide Pbr5

Pbr5 Lewis Structure Lewis Structure Of Pbr5 Phosphorus Pentabromide Draw Lewis Structure For Pbr5 Youtube

Pbr5 Lewis Structure Phosphorus Pentabromide Youtube
Molecular Geometry Ck 12 Foundation

Pbr5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Phosphorus Pentabromide Pbr5 Pubchem

Phosphorus V Oxybromide 95 7789 59 5

What Is Pbr5 Lewis Structure Study Com