Sci2 Lewis Structure Molecular Geometry
Also the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell. Lewis Dot Structure for SbCl5 and Molecular Geometry - YouTube.
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
Hybridization of SO42-sp3 hybridization.

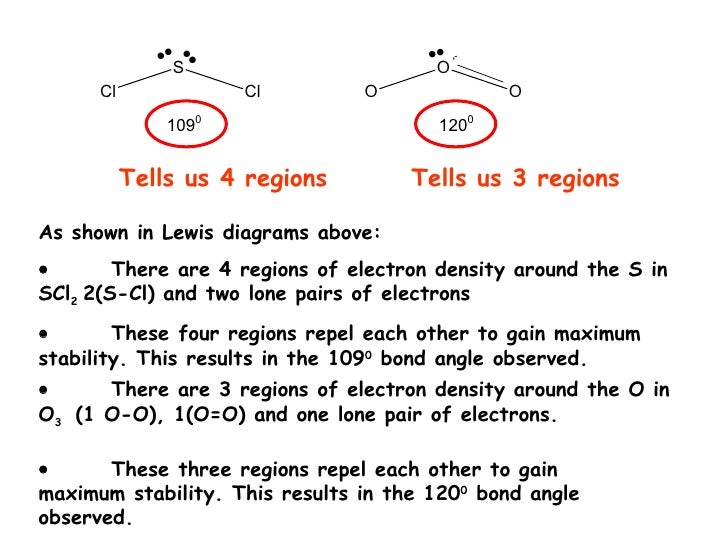
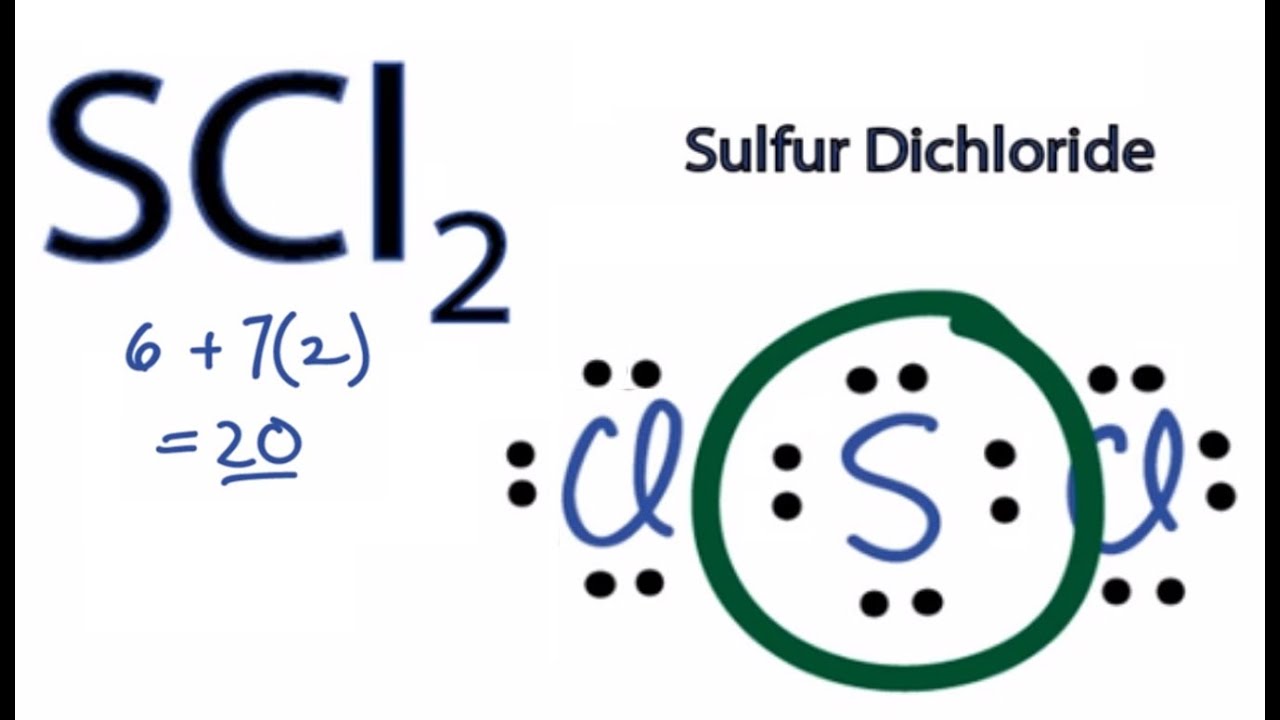
Sci2 lewis structure molecular geometry. Lewis structure is the placement of the electrons around the atoms of a compound. The initial step is to calculate the valence or outermost shell electrons in a molecule. This gives us a linear shape initially.
However there are two lone pairs of electrons on each Oxygen atom. For the SCl2 Lewis structure use the periodic table to find the t. And for the Carbons each Carbon has four single bonds so thats 8 valence electrons and thats an octet for the Carbon.
And as per VSEPR theory these lone pairs try to repel each other which distorts the molecules shape. The compound SiCl 4 itself is very dangerous in nature. Indicate the total number of valence electrons in the following ions.
With this information molecular geometry hybridization and molecular orbital diagram can be studied further. VSEPR theory helps us determine the molecular geometry of any given molecule. Start with the molecules Lewis structure which is drawn like this.
We also look at the molecular geometry bond angles and electron geometry for SbF. Here two Oxygen atoms are forming bonds with Hydrogen atoms. The fundamental premise of the theory is that electron pairs whether contained within bonds or in lone pairs repel each other because of their like charges.
As this molecule has many applications in various industries today it is vital to know its Lewis Structure Molecular Geometry and more. Before quickly jumping into the lewis structure of SO2 lets have an abrupt discussion regarding the usefulness of the lewis structure and the steps to draw it. Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms.
Lewis Structure is a 2D diagrammatic representation of the arrangement of electrons note. No of Valence Electrons in the molecule. NO3 Lewis Structure Molecular Geometry and Hybridization.
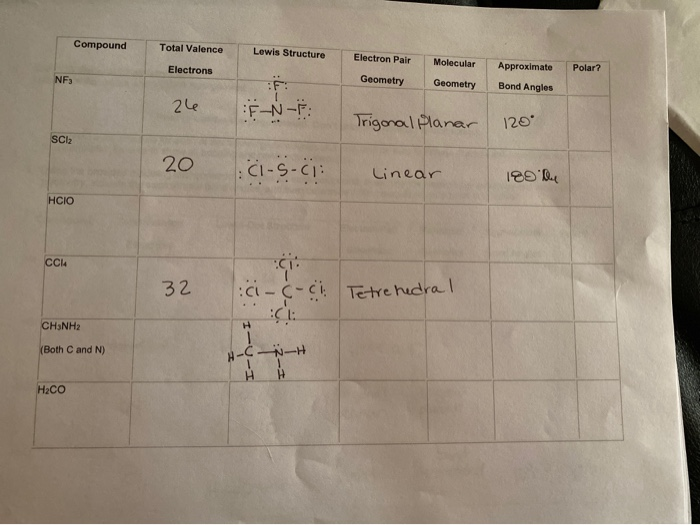
NHA PO3- NO2 H3O here to search 13 WRITTEN ASSIGNMENT 3. SiCl4 NF3 CO2 HNO SCI2 H2O 92 Molecules and Charge 2. To understand it in detail we have to first get acquainted with the concept of Lewis Structure.
It is important to remember that Lewis structures are not meant to convey geometry so it would be wrong to assume that the molecule is linear just by looking at this particular Lewis structure. As seen in the Lewis structure above the Chlorine atoms repel each other. H2O2 Molecular Geometry.
There are three lone pairs present on the central atom of ICl2- lewis structure. SCl2 Molecular Geometry and Shape. Aluminium chloride is corrosive to tissue and toxic by ingestion.
SCl_2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. Once the Lewis structure of a molecule or ion is determined the 3-D shape of the molecule can be determined. A step-by-step explanation of how to draw the SbF5 2- Lewis Dot Structure.
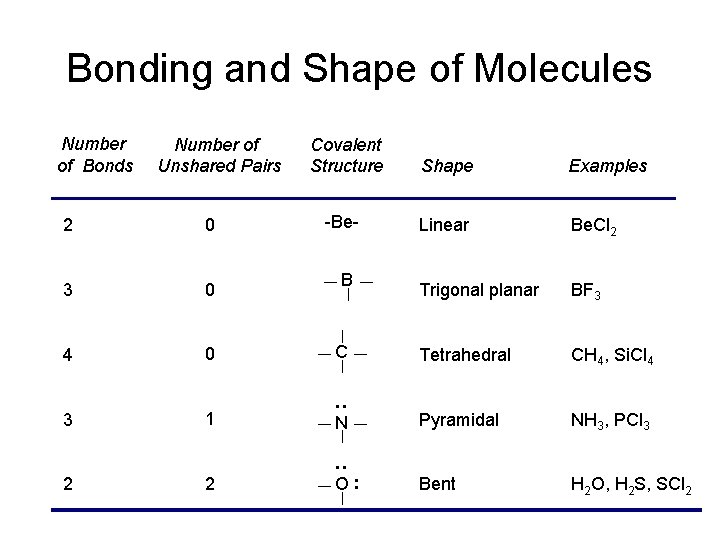
Approximately 1095 Molecular Geometry of SO42-Tetrahedral. Therefore SCl2 has a Bent molecular geometry and a tetrahedral shape in nature. ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position.
Draw Lewis structures for each of the following molecules. This is in accordance with the VSEPR theory. The Valence Shell Electron Pair Repulsion theory or VSEPR theory is one useful theory for predicting the geometries of molecules.
Valence electrons inside a molecule. It gives us a graphical sketch with electron-dot notations for us to grasp the process in a simple manner. In the following structures one atom has a nonzero formal charge.
Now lets go through the method of drawing lewis structure. SCl_2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. In this tutorial we will study Aluminium chloride AlCl3 lewis structure molecular geometry hybridization polarity bond angle etc.
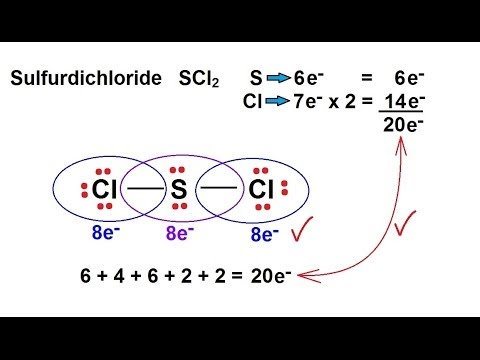
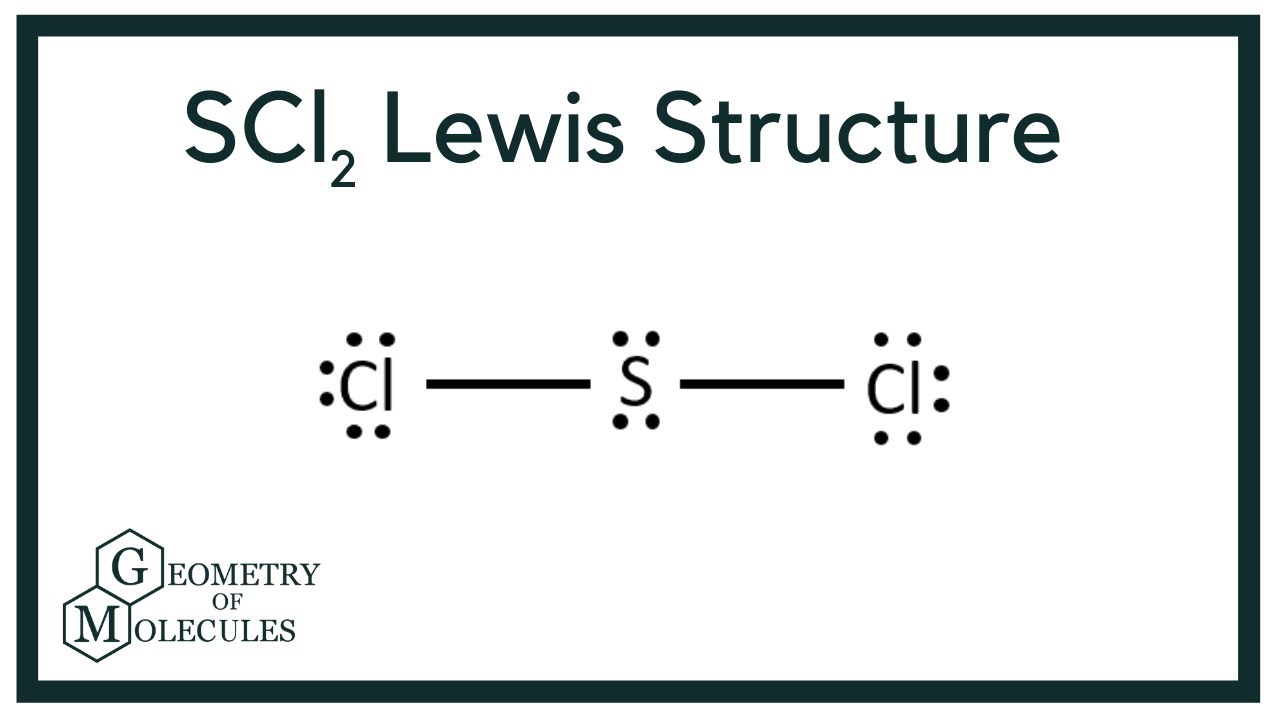
The structure determines how sharing of the valence electrons is taking place and whether a single double or triple bond is forming. It sometimes appears yellowish in color due to the presence of contaminants. A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride.
There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure. It is powerful lewis acid and capable of reversible changing from polymer to monomer at mild temperature. This structure benefits us to know about the kind of bonds and the number of bonds that form the compound.
However upon the addition of the two lone pairs on Sulfur the molecular geometry becomes bent. All 20 valence electrons 6 from S and 7 from each Cl atom are accounted for by the Lewis structure. In this blog post we will go through all the details.
SCl2 lewis structure contains one sulfur and two chlorine atom. Locate that atom and indicate the charge. Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewiss diagram and chlorine is spaced evenly around it.
It crystallizes in a face-centred cubic structure which has an octahedral coordination geometry each Mg2 is surrounded by six O-2 ions and each O-2 ion is likewise surrounded by.

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

What Is The Electron Pair Geometry And Mol Clutch Prep

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
Vsepr Theory L Types Of E Pairs Bonding
Bond Angle Of Scl2 Lewis Structures

Warm Up Name Or Write The Following Compounds Ppt Download
2 Consider The Molecule Scl2 I Sketch The Lewis Chegg Com
Journal Entry Draw Lewis Structures For The Following

Does Scl2 Have A Dipole Moment Clutch Prep
Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube
Compound Total Valence Lewis Structure Electron Pair Chegg Com
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Bonding Molecular Shape Structure By Dr Fawaz Aldabbagh

The Lewis Diagram For Scl2 The Electron P Clutch Prep

Section 6 5 Polar Bonds And Intermolecular Forces Ppt Download

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape