Sif4 Total Valence Electrons
Fluorine group 7--7 valence electrons but we have 4 of those so were going to multiply that by 4. Total number of valence electrons in SF4 number of valence electrons in sulfur number of valence electrons in fluorine 6 28 34 valence electrons Now that we know the total number of valence electrons it would become easy for us to understand the bond formation between the atoms and the complete arrangement of the molecule too.

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora
Species having electrons less than 8 or more than 8 will not obey the octet rule.

Sif4 total valence electrons. Thus the Lewis structure is as in the first image below. Now draw single bonds around the central atom connecting Fluorine atoms. Hence the silicon atom has four valence electrons whereas the fluorine atom has seven valence electrons.
Calculate the total valence electrons in the molecule. Point group T d. S has 4 valence electrons plus 1 for each Si-F single bond.
Thus the Lewis structure is as in the first image below. Four fluorine atoms have total 28 electrons. Total 8 electrons four pairs.
Vapor is heavier than air. So we have 32 valence electrons to work with. If complete octets cannot be formed when using only single bonds between atoms it is necessary to draw multiple bonds.
Total Valence Electrons Formula SiF4 Bond Polarity AEN olal Valence Electrons Pormula PH3 Bond Polarity AEN 10 Total Valence Electrons Formula BF4 Bond Polarity ΔΕΝ Total Valence FormulaElectrons SbCl6 Bond Polarity AEN 12 Total Valence Formula TeF4 Bond Polarity ΔΕΝ. A single chemical bond takes u one valence electron from each atom. Under prolonged exposure to heat the containers may rupture violently and rocket.
Distribute rest of electrons. Each single bond is made of 2 electrons. 1 SiF4 Four bond pairs no lone pair.
The silicon and fluorine atoms belong to group 14 and group 17 respectively. Silicon tetrafluoride contains one silicon atom and four fluorine atoms. Very toxic by inhalation.
In its atomic state all those electrons are available to bond and. The spectrum reveals vibrational structure in three of the four accessible electron bands. It means it has total of 8 electrons.
Jmol_Canvas2D Jmol jmolApplet0 x loadScript jsmolj2scorepackagejs. For SiF4 all the Si electrons are in bonds but each F atom has 6 electrons still available. On the periodic table Silicon is in group 4 sometimes called 14 so its got 4 valence electrons.
The He I induced valence electron spectrum of SiF 4 is presented and the energies of the bands are reported. Total 8 electrons are used up in making 4 single bonds. 8 e total valence electrons1 atom of O6 valence e3 atoms of H1 valence e1 valence e17 e When is it necessary to draw a multiple bond in an electron-dot formula.
Silicon tetrafluoride appears as a colorless nonflammable corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Structure tetrahedral class AX 4. A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure Silicon TetrafluorideFor the SiH4 structure use the periodic table to find the tota.
Nitrogen is in 5A of the PT and so has 5 valence electrons. Therefore silicon tetrafluoride consists of 4 74 32 valence electrons in total. For SiF4 all the Si electrons are in bonds but each F atom has 6 electrons still available.
Nitrogen is in 5A of the PT and so has 5 valence electrons. Is SeF4 trigonal bipyramidal. Put Silicon in center and arrange fluorine atoms on the sidesput a pair of electrons connecting each fluorine with oxygen and the remaining four electrons on fluotrine atoms.
It means it has total of 8 electrons. And that is 4 plus 28 is 32. Only the band at 195 eV has been reported previously to exhibit vibrational progressions.
How many valence electrons do you have left in SeF4. In silicon it has four valence electrons Group number will be same as number of valence electrons and Fluorine atom have 7 valence electrons.

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules
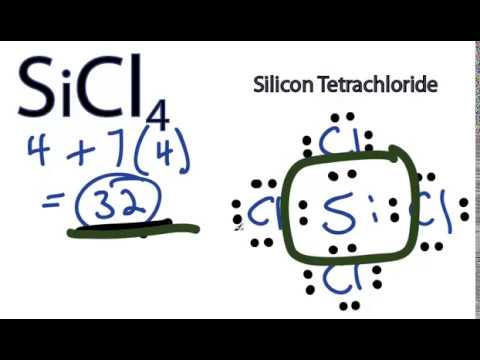
Sicl4 Lewis Structure How To Draw The Lewis Structure For Sicl4 Youtube

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Xef6 Lewis Structure How To Draw The Lewis Structure For Xef6 Youtube

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Sif4 Molecular Geometry Bond Angles Electron Geometry Youtube

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium
