What Is The Bond Angle Of Pcl3
Predict The Geometry And Bond Angles Of PCL3 And ICL4-This problem has been solved. Because of the electron pair the bond angle in PCl3 is.

Arrange The Following Acl8 Species In Order Of Decreasing Cl Clutch Prep
Why BH3 exist but not BCl3.
What is the bond angle of pcl3. What is the bond angle of pcl3. But the bond pair-bond pair repulsion increases by the increase in size of the bounded element bigger atom means bigger electron density around it. Since it has 4 groups on it it is expected to follow a bond angle of 1095 bond angles of tetrahedral structure however it deviates due to the presence of lone pairs.
Expected bond angles will appear as. BH3 is an electron deficient compound which has an empty p orbital or we can say 6 electron in its outermost shell. A PCl3 Is contains three covalent bond which are formed by equal sharing of electron in between phosphorus atom and chlorine atom hence its a covalent molecule.
The bond pair-bond pair repulsion increases more than the lone pair-bond pair repulsion when going down a group. Structure of PF 3 In case of PCl 3 the size of Cl is larger as compared to F. A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond anglesLooking at the PCl3 Lewis structure we can see that the.
Thus the bond angle is larger as it has to face lesser repulsion in case of P-F-P bond angle. As the size of Hydrogen is small therefore it facilitates the dimerisation of BH3 through 3 entered 2 electron bond. The bond angle marked a in the following molecule H4C2O2N is about _____ 120.
If the number of rodsbonds is 2 then the shape is linear and angles are 180 degrees. PCl3 has a bond angle of 103 degrees. The 3 bonds lie in the same plane making an angle of 120 degrees with one another.
General structure of the molecules as PX 3 X halogen will appear as. If the number of rodsbonds is 3 then the shape is triagonal planar and angles are 120 degrees. According to the Colby Chemistry Database the bond angle of cePBr3 and cePCl3 are around 101 degrees but the bond angle of cePH3 is 92 degrees.
The remaining 3 bonds are equatorial in nature. PCl3 Molecular Orbital MO Diagram In a molecular orbital diagram of PCl3 we can see 3 bonding orbitals which will be occupied. Previous question Next question.
Here since you have two lone pairs you could say the same exact thing again its electronic geometry is still AX4 ideally it should be 1095 but the lone pairs being there make it less than 1095. And the lone pair bond pair repulsion plays a bigger role in deciding the bond angle. Both the bonds make an angle of 90 degrees with the plane.
PCl3 PBr3 PH3 There was a previous question on Stack Exchange about the bond angle difference in cePF3 and cePH3 here. PCl3 has distorted structure due to the presence of lone pairs of electrons. The ideal bond angle is 1095 but because that lone pair is there all youd have to really say is you would expect the bond angle to be less than 1095.
So due to bp-bp the bromine bond angle will be more than chlorine. 5 rows Looking at the PCl 3 molecular geometry it is trigonal pyramidal with a bond angle of approx. BCl3 exist but BH3 does not.
PCl3 is sp3 hybridized. So in this case the bonds are longer and bond pairs have enough space between them. We can see there are 4 things around the central atom.
The decrease from the ideal bond angle of trigonal pyramidal compounds 109 degrees is due to repulsion between the lone pairs on phosphorus. Predict The Geometry And Bond Angles Of PCL3 And ICL4-Question. How do we predict bond angles.
3 Chlorines and a lone pair of electrons. What bond angle is NI3. PCl3 ii CCl4 iii TeCl4 iv XeF4 v SF6 For which of the molecules is the molecular geometry shape the same as the VSEPR electron domain arrangement electron domain geometry.
100 8 ratings Hybridization sum of sigma bonds present total number of lone pairs In PCL3 3 sigma bonds and one lone pair for P view the full answer. Predict the geometry and bond angles of PCL3 and ICL4-Expert Answer 100 3 ratings Previous question Next question. PF3 and PH3 but the accepted answer mentions back bonding as.
If the number of rodsbonds is 4 then the shape is tetrahedral and angles are 109 degree 28 minutes.

Pcl3 Lewis Structure And Molecular Geometry Youtube

Pcl3 Molecular Geometry Shape And Bond Angles Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

How Can The Molecular Geometry Of Phosphorus Trichloride Be Described Quora
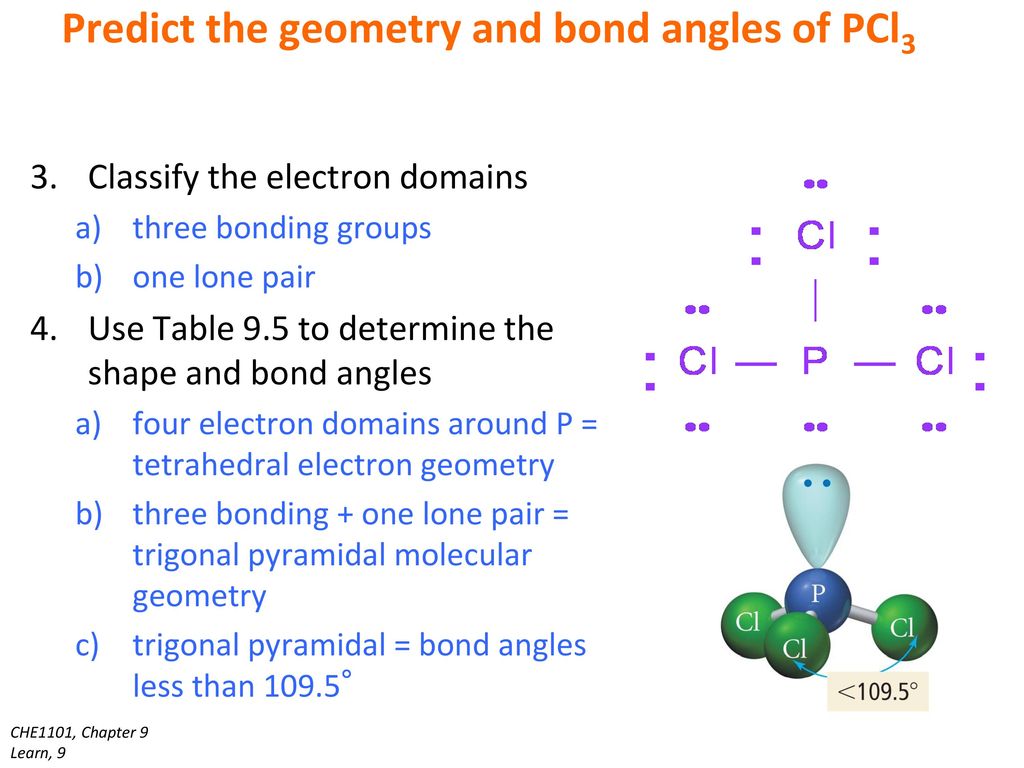
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

What Is The Molecular Shape Of Pcl3 Quora

What Is The Molecular Geometry Of Pcl3 Study Com

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pocl3 Molecular Geometry Shape And Bond Angles Youtube

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

What Is The Bond Angle Of Pcl3 Quora

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Is Pcl3 Polar Or Nonpolar All About Pcl3 Polarity Structure
Answer In Organic Chemistry For Tj 92420

Structure Of Nh3 Ph3 Pcl3 And Pcl5 Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization