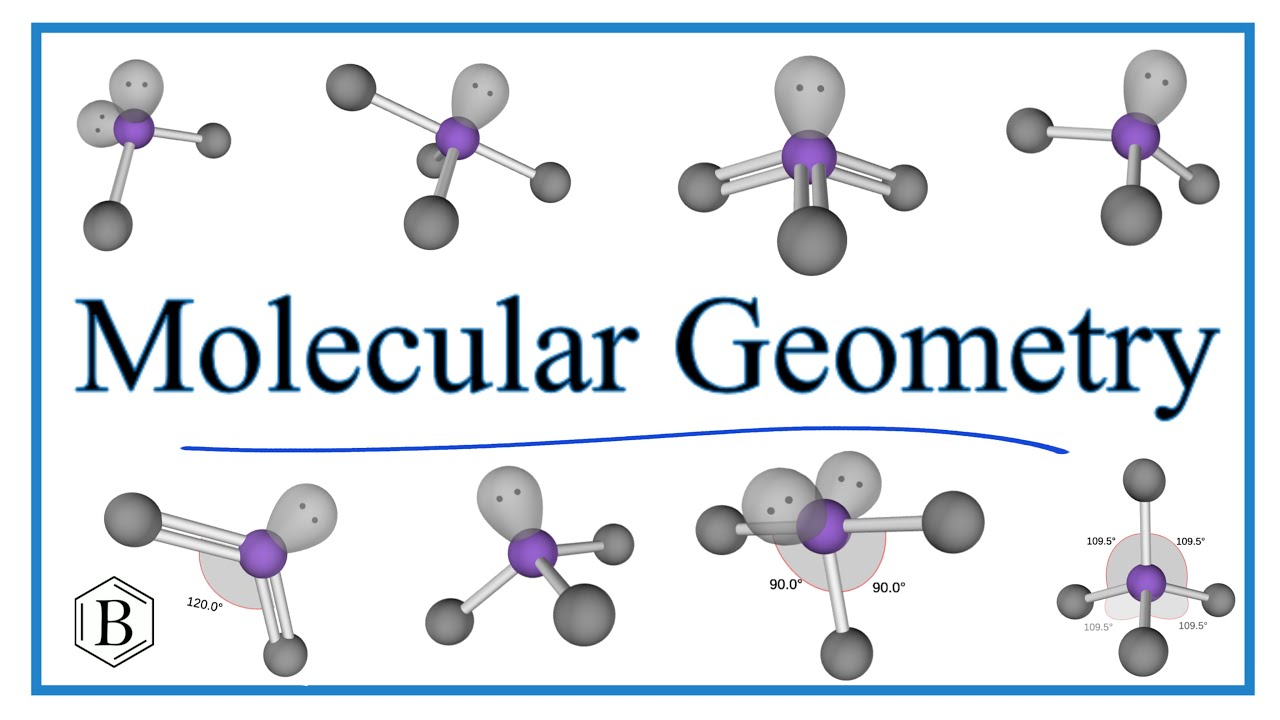
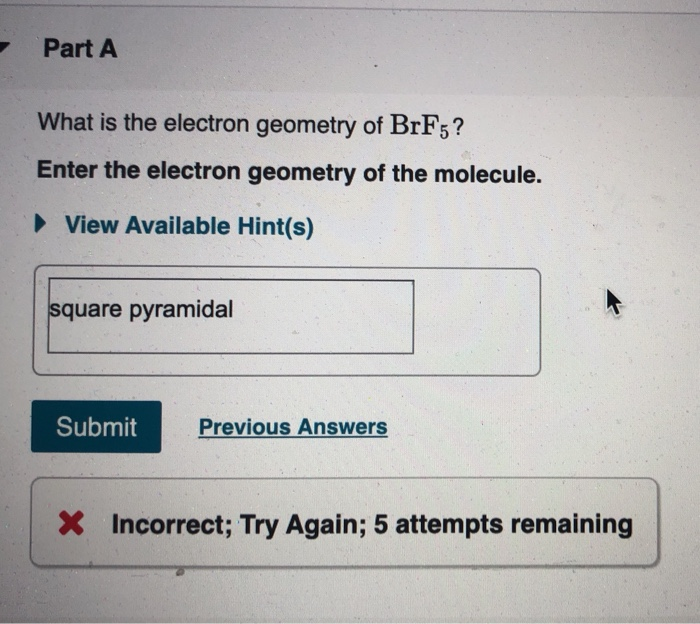
What Is The Electron Geometry Of Brf5
In IF5 considering I to be the central atom it has 7 valence electrons. Bromine is the least electronegative well put that in the center and then well put 5 Fluorines around the outside.

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
What is the molecular geometry of XeF2.

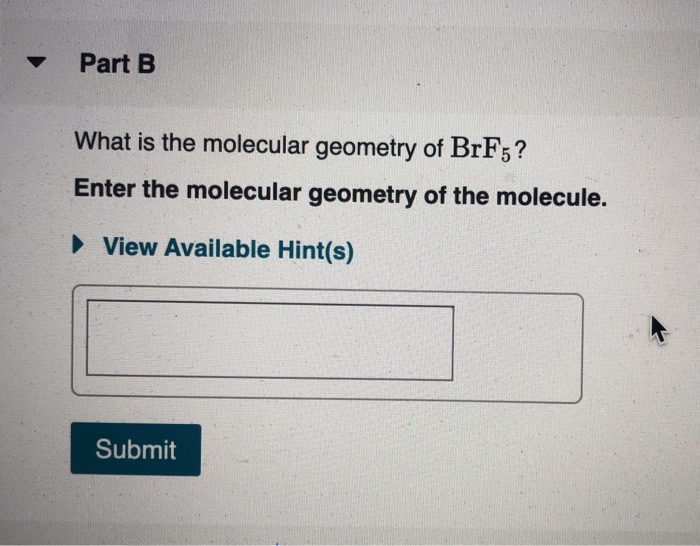
What is the electron geometry of brf5. View Available Hint s Submit Part D Which choice best describes the polarity of BrF5. The bond angle of BrF5 is 90º. Our videos prepare you to succeed in your college classes.
For BrF5 we have a total of 42 valence electrons. Because the core central atom bromine has five Br-F bonds with the surrounding fluorine atoms. What are BrF5 electron and molecular geometry.
What is the electron geometry of NCl3. What is the molecular geometry of SiO2. Asked by ss11232428 30th Aug 2015 0730.
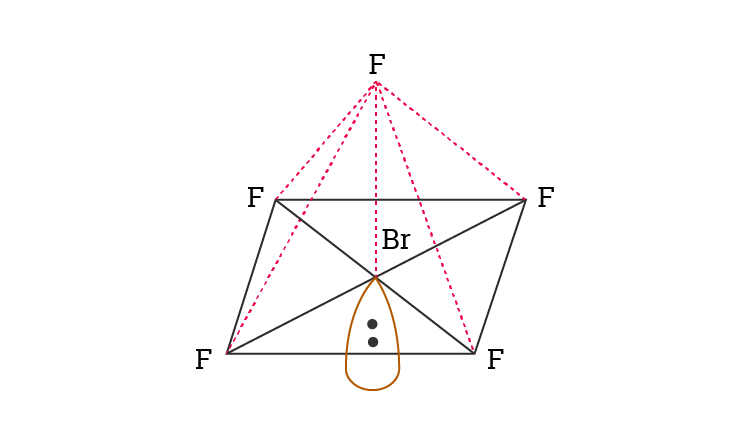
The molecule is polar and has nonpolar bonds. BrF5 lewis dot structure has 10 sharing electrons and 32 non-sharing electrons. The shape of BrF 5 molecule is square pyramidal.
The molecular geometry of BrF 5 is Square Pyramidal Fig 1 a and the Number of electron groups is 6. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. The difference between both the values is 102 which is greater.
The angle will be slightly less than 90 0. Give the molecular geometry and number of electron groups for BrF 5. 5 electron groups 2 lone pairs 180.
Read More About Hybridization of Other Chemical Compounds Hybridization Of XeF4. Which type of hybridization leads to a bent what do the properties of molecular substances What is the difference between the electron domain. The Correct Answer is.
The molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution. What is the molecular geometry of XeF4. What is shape of XeF4 xeo3.
According to the VSEPR theory the shape of the molecule is determined by both the total number of electron pairs bonding and non-bonding around the molecules central atom and the orientation of these electron pairs in the space around the central atom. View Available Hint s The molecule is polar and has polar bonds. BrF 5 molecular geometry is said to be square pyramidal with a bond angle of 90 o each.
The geometry of XeF4 is a square planar with symmetric electron reigon distribution. The central Br atom has six electron domains so the electron geometry is octahedral. Therefore XeF4 molecular geometry is square planar.
What is the molecular geometry of BrF5. According to Tutor Homework the polarity is best found by first drawing the Lewis dot structure for BrF5. Molecular geometry is that kind of geometry that shows the three-dimensional representation of any given molecule.
Thus the electron-pair geometry is tetrahedral with three of the corners occupied by the bonding pairs of electrons. What is the molecular geometry of NCl3. Enter the molecular geometry of the molecule.
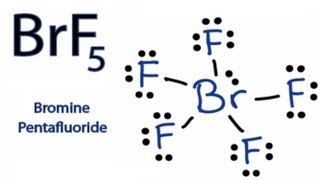
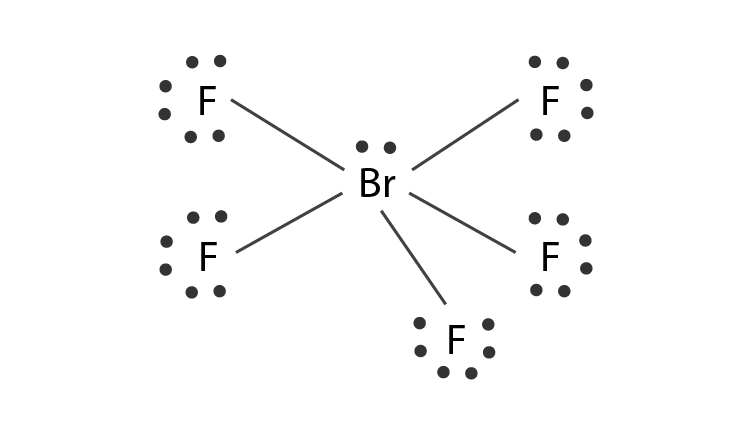
From the Lewis dot structure of BrF5 it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 296 and 398. In the same plane each F-Br-F bond forms a 90-degree angle. What is the Lewis structure of BrF5.
The molecule is nonpolar and has polar bonds. What are the bond angles for octahedral molecular geometry. What is the electron-domain charge-cloud geometry of BrF5.
What is the O-S-O bond angle in SO 3. What is the molecular geometry of BrF5. And 2 electrons form a lone pair of electrons.
This is the BrF5 Lewis structure. Why does IF5 exist. What is the electron geometry of BrF5.
What type of compound is IF5. What are the bond angles for linear molecular geometry. 5 of them form Sigma covalent bonds with 5F atoms.
Let us help you simplify your studying. CHM CH 5 6. The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral.
O The molecule is nonpolar and has nonpolar bonds. What is the formula for phosphorus triiodide. YOU MIGHT ALSO LIKE.
XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other. Molecular geometry and electron-group What the molecular shape geometry of CHClO. The molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution.
BrF5 has a square pyramidal molecular geometry and an octahedral electron geometry according to the VSEPR theory. It provides the shape concerning the bond length bond angles. Our videos will help you understand concepts solve your homework and do great on your exams.
The O-S-O bond angle in SO 3 is 120 with trigonal planar geometry Fig 1 b. The molecular geometry of the BrO3 - ion is _____. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back.
True or False. The electron geometry is octahedral and the hybridization is sp3d2.

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Bond Angles In Brf5 Chemistry Stack Exchange
Part A What Is The Electron Geometry Of Brf5 Enter Chegg Com
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
How To Determine The Molecular Geometry Of Brf5 Quora

What Is The Molecular Geometry For Brf5 A Clutch Prep
Part A What Is The Electron Geometry Of Brf5 Enter Chegg Com

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

What Is The Electron Geometry Of Brf5 Enter The Electron Ge Clutch Prep

Answer What Is The Electron Domain Charg Clutch Prep
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

Consider The Given Lewis Structure For Brf5 What Is

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube