What Is The Electron Geometry Of Pbr3
It is composed of one gallium atom and three bromide atoms. American Conference of Governmental Industrial Hygienists.

Pin On Medical School Optometry School Tipa
Phosphorus halides including phosphorus trichloride and phosphorus tribromide have been considered as potential replacements for Halon 1301 as fire suppressants.

What is the electron geometry of pbr3. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. So according to the VSEPR chart H3O has trigonal pyramid as its molecular shape and tetrahedral as its electron geometry. PBr3 is a molecular compound there are three single bonds and a lone pair around the phosphorus atom making up the octet.
Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3. Phosphorus Trichloride PCl3 has a total of 26 valence electrons. It has sp3 Hybridization and the bond angle is approximately 1095.
5 for P 7 for each bromine atom respectively. Our videos prepare you to succeed in your college classes. Given is the Lewis structure of phosphorus tribromide eqPBr_3eq which has a pyramidal geometry.
An explanation of the molecular geometry for the IBr3 ion Iodine tribromide including a description of the IBr3 bond angles. The phosphorus atom is eqsp3eq. D The electron-pair geometry is.
From here you can easily see that there are three Br atoms covalently bonded to the central P atom with one single bond each. 5 73. In the phosphorus tribromide the central atom is P and is forms three sigma bonds with each bromine atom respectively.
Let us help you simplify your studying. Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of phosphorus tribromide PBr3. Predicting molecular geometry ¾To determine the molecular geometry Find number of valence electrons Draw the Lewis structure Count the number of electron pairs bond pairs and lone pairs but count multiple bonds as one pair Arrange electron pairs to minimise repulsion Name the geometry from the atom positions.
It is used in the laboratory for the conversion of. Part 31 Pt What Is The Molecular Geometry Of PBr3. You get a tetrahedral only when there are 4 atoms around a center one or.
In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom. What is the molecular geometry of PBr 3. Our videos will help you understand concepts solve your homework and do great on your exams.
Within a molecule of gallium tribormide there are three bonding electron pairs and zero lone pairs of electrons. Part 4 1 Pt Which Of The Following Best Describes The Bond Angles In PBr3. Phosphorus has an atomic number of 15 and therefore has a valency of 5.
From the above chart we can see that hydronium ion is a AX3E type molecule A central atom X bonded atom E lone pair on A. The molecule is trigonal pyramidal-shaped and is a polar molecule. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back.
In the case of Br it belongs to the family of halogens and consists of seven valence electrons. Phosphorus tribromide is a liquid whose fundamental unit is the PBr₃. The molecular geometry for BBr3 is Trigonal Planar.
There are a total of 26 valence electrons for PBr3. Part 1 1 Pt See Periodic Tab Draw The Bond-dot Lewis Diagram Of PBr 3. Boron tribromide is used as a catalyst in the manufacture of diborane ultra high purity boron and semiconductors.
The electron geometry for the. In other words one P atom utilises three of its five outer electrons to form such bonds each Br atom contributes with one electron each. Valence electrons of Phosphorus Valence electrons of Chlorine.
There is 26 total valence electrons. See full answer below. There has been one report of PBr3 effectiveness on real-scale flames indicating an effectiveness two orders of.
Total valence electrons in a single molecule of PBr3 5 73 5 21 26. What is the molecular geometry of IF4 plus. Phosphorus tribromide or Pbr3 molecule consists of a phosphorus atom and three atoms of bromine.
Phosphorus tribromide is a colourless liquid with the formula P Br 3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. Select A Tool To Begin Drawing Part 2 1 Pt What Is The Electronic Geometry Of PBr3.
Total number of valence electrons of PCl3. The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral.

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Chemistry Molecular Geometry

Common Silyl Ether Protecting Groups Organic Chemistry Study Chemistry Lessons Ethereal

Lewis Dot Structures For Polyatomic Ions Clear Simple Practices Worksheets Chemistry Worksheets Worksheets

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Teaching Chemistry Chemistry Worksheets Chemistry Education

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Pin By Sarah Hawkins On Chemistry Class Molecular Geometry Teaching Chemistry Chemistry Class

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Chemistry Chemistry Lessons
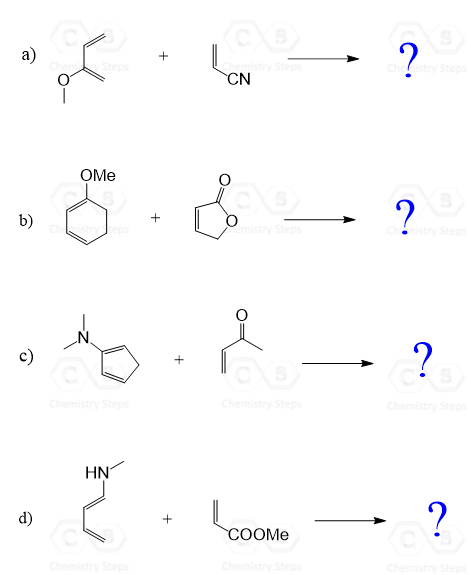
Diels Alder Regiochemistry Organic Chemistry Questions Chemistry Organic Chemistry

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Teaching Chemistry Chemistry Worksheets Chemistry Education

The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry Organic Chemistry Chemistry Chemistry Lessons

Molar Mass Of Nh4cl Ammonium Chloride In 2021 Molar Mass Molars Molecules