What Is The Formal Charge Of Hno3
Nitric acid is a nitrogen oxoacid of formula HNO3 in which the nitrogen atom is bonded to a hydroxy group and by equivalent bonds to the remaining two oxygen atoms. Show work By signing up youll get thousands of step-by-step.

Consider The Lewis Structure For The Nitri Clutch Prep
In the Lewis structure for HNO 3 there are a total of 24 valence electrons.

What is the formal charge of hno3. What is the formal charge on the phosphorus P atom in PCl4. Answered 2 years ago Author has 137 answers and 1307K answer views The formal charge of N in nitric acid is 5. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
Think of it as H and NO3-. O N OO. N in HNO3 carries a ve charge the O bonded to this N by a coordinate or dative bond gains -ve chargeThe charges on N O are nothing but formal charges.
The whole nitrate ion carries a total charge of minus 1 when combining the charges of the one nitrogen atom and three oxygen atoms. Ol H 3. 1 2 0 -1 -2.
For chlorate you would expect to leave four single bonded oxygens to the chlorine however we are left with a formal charge of 3 on the chlorine and -1 on each of the oxygens. The inner core of the electrons is associated with just 6 electrons instead of the 7 that are needed for electrical neutrality meaning it has a positive charge overall. How do the P-Ci single bond lengths in PCl5 PCl4 and PCl6- generally compare.
Identify the type of bonding the central atom undergoes in PCl5 and PCl6-. Therefore we form double bonds until the formal charge is removed and are left with only a formal charge of. The overall charge on the ion is still 1 but there is again formal charge separation in our representation of the anion.
Formal charge for C2H2. Formal charge for C2H4. In this case the formal charges will be closer to zero if you place a double bond beteween the Nitrogen atom and the Oxygen atom without the H attached.
Lewis structure of nitric acid There is. Check the formal charges to be sure that each atom has a formal charge of zero. The formal charge of nitrogen in the compound NO3 is plus 1.
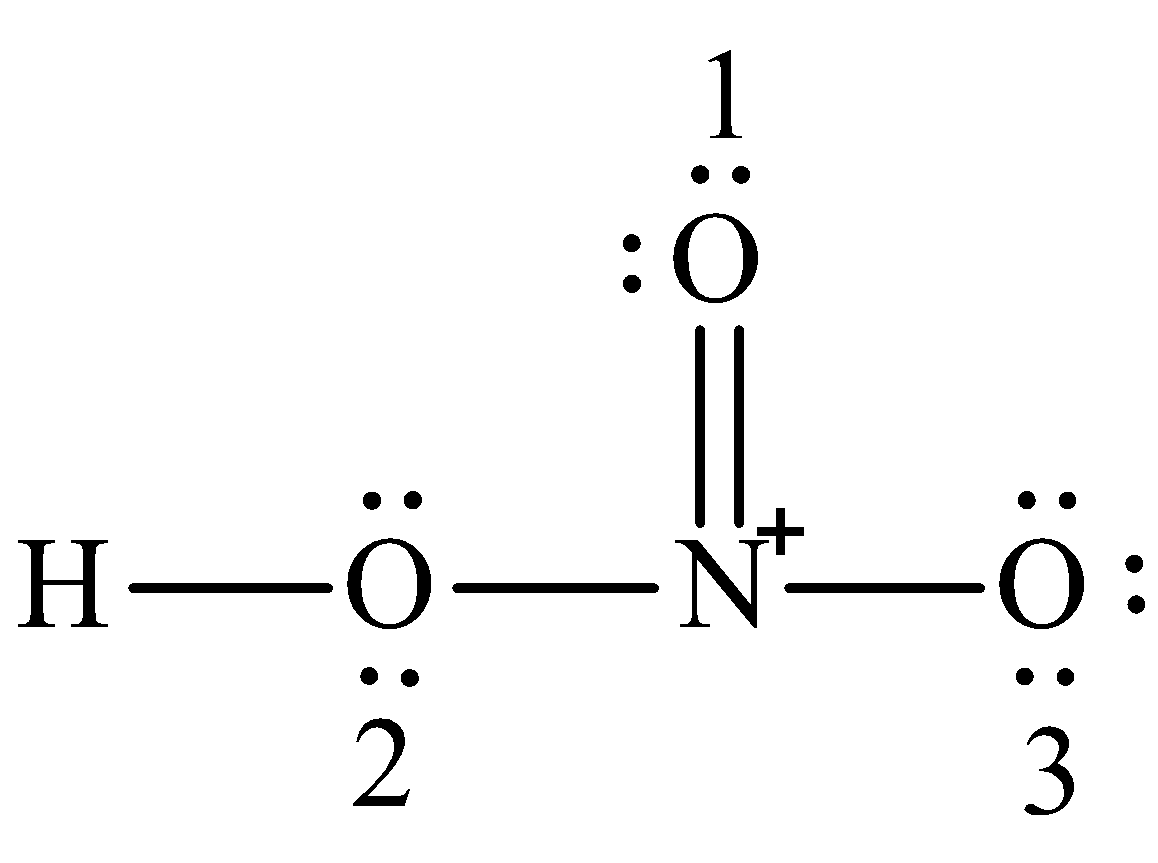
It is a conjugate acid of a nitrate. The total charge is -1. Answer to What is the formal charge of N in HNO_3 as seen below.
In order to calculate the formal charges for NO3- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding ele. Formal charge v -u - 12 be. Since the nitrogen atom has only 6 electrons total it therefore has a formal positive charge.
In NO3- the three Oxygens are -2 for a total of -6. That leaves 5 to make everything equal. The formal charge is found by subtracting the number of lone electrons and half the number of bonded electrons from the total number of valence electrons.
In order to calculate the formal charges for N2O well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec. Where v valence electrons on the atom. Formal charge for HNO3.
The nitrogen atom has no lone. It has a role as a protic solvent and a reagent. In order to calculate the formal charges for HNO3 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding ele.
There are 3 formal charges on the nitrate ion. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. What is the formal charge on the chlorine Cl atom in PCl₄.
Write Lewis Structure Of The Hno3 And Show Formal Charge Class 12 Chemistry Cbse

Assign Formal Charges To All Atoms In The Following Chegg Com
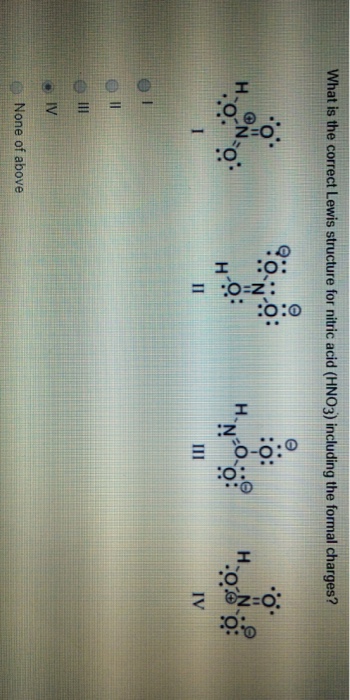
What Is The Correct Lewis Structure For Nitric Acid Chegg Com

Write Lewis Structure Of The Hno3 And Show Formal Charge On Each Atom
What Is The Formal Charge Of N In Hno 3 As Seen Chegg Com

Write Lewis Structure Of The Following Compounds And Show Formal C
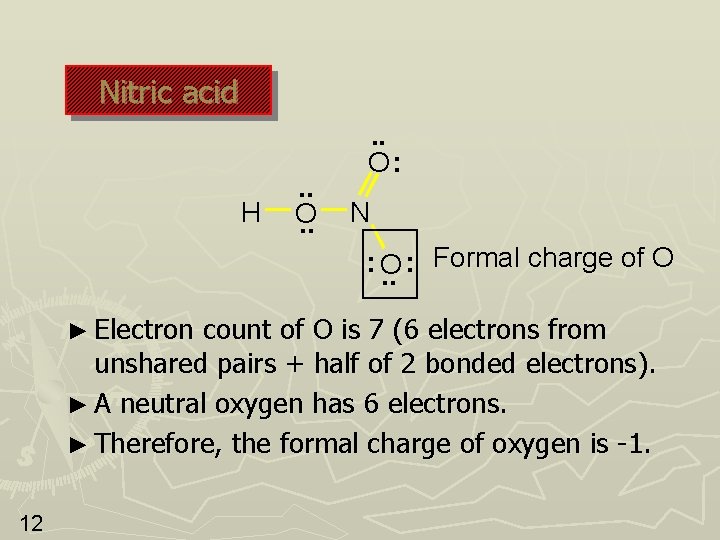
Resonance And Formal Charge 1 Resonance And Formal
Write Lewis Structure Of The Following Compounds And Show Formal Charge On Each Atom Hno3 No2 H2so4 Sarthaks Econnect Largest Online Education Community

How To Calculate The Formal Charges For Hno3 Nitric Acid Youtube
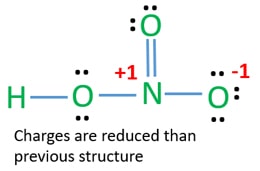
Hno3 Nitric Acid Lewis Structure

Calculating No3 Formal Charges Calculating Formal Charges For No3 Chemistry Classroom Science Chemistry Chemistry
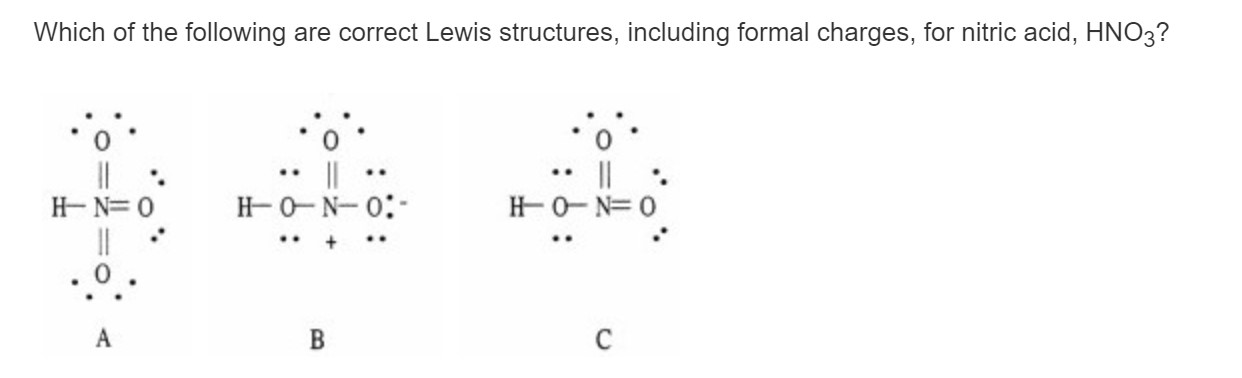
I I Know Why B Is Correct But Why Is C Wrong Chegg Com
What Are The Lewis Structures For Hno3 Quora

Hno3 Lewis Structure How To Draw The Dot Structure For Hno3 Chemical Bonding
Solved Which Of The Following Are Acceptable Lewis Structures Including Formal Charges For Nitric Acid Hno3 Course Hero

1 Draw The Lewis Structure Of Nitric Acid Hno 3 That Minimizes Formal Charges Assign Lone Pairs Radical Electrons And Atomic Charges Where Appropriate 2 Calculate The Electrons Required Er Study Com

The Lewis Structure Of Hno3 Chemistry Stack Exchange

What Is The Formal Charge On Atom In Carbonate And Nitric Acid
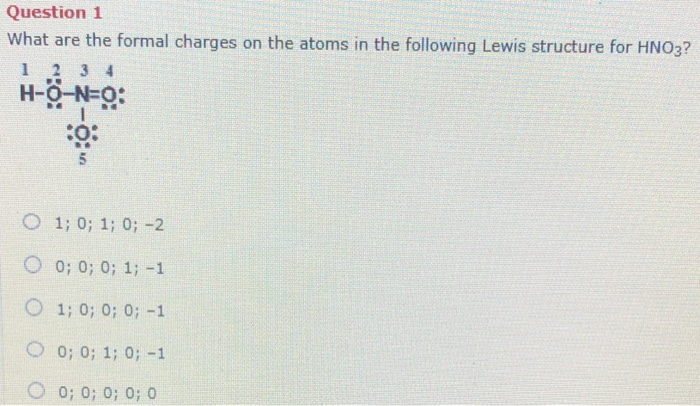
Question 1 What Are The Formal Charges On The Atoms Chegg Com