Xef4 Lewis Structure Explained
So xenon oxytetrafluoride will have a total of. The XeF4 has a solid white appearance and has a density of 4040 g cm3 in a solid form.

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
XeF4 Xenon tetrafluoride Lewis Structure.

Xef4 lewis structure explained. Explain the differences and similarities. Write the bond lengths and bond angles in each case. This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals.
In the best Lewis structure for XeF4what is the formal charge on the F atom. Chemistry questions and answers. B-1 C 42 D 0 A 1.
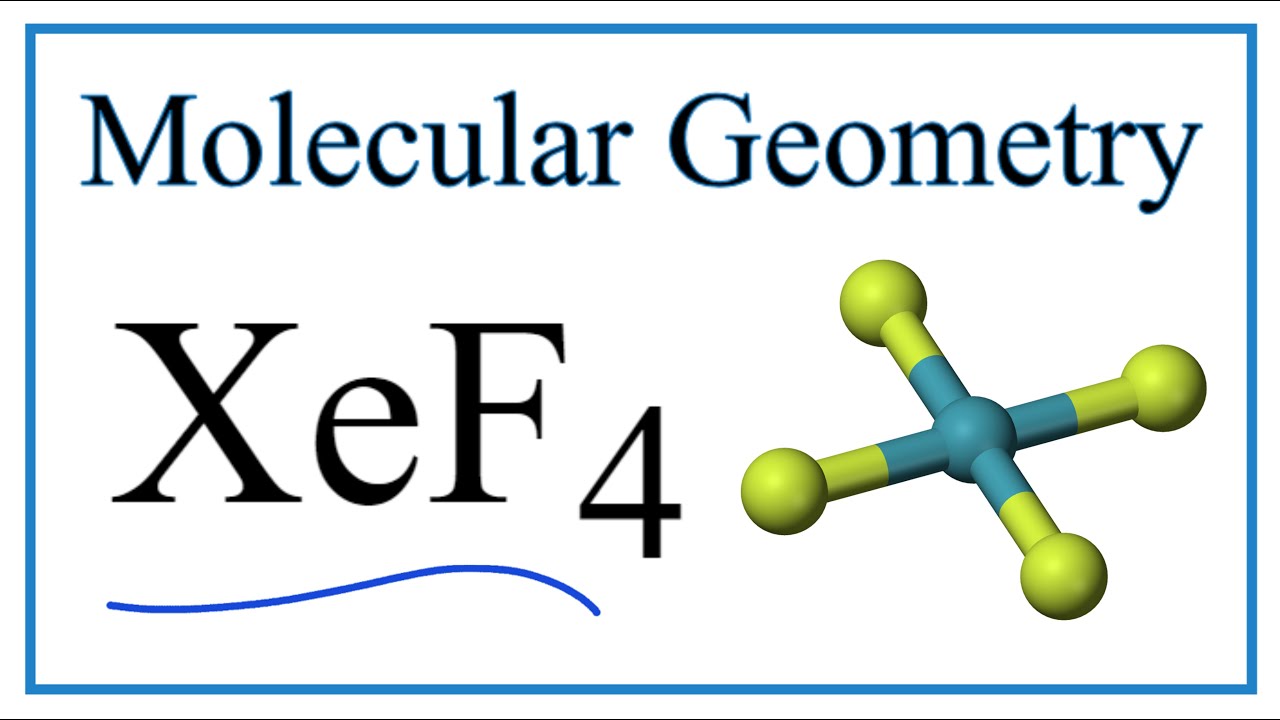
Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1. Molecular Geometry of XeF4.
What is the Lewis structure of XeF4. In the best Lewis structure for XeF4what is the formal charge on the F atom. Draw and explain the Lewis structure for the molecule eqXeF_4 eq which does not.
The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and Fluoride atoms. Xe 2 F2 - XeF4. The electron structure that shows is called the Lewis Structure of XeF4.
Find valence e- for all atoms. Draw and explain the Lewis structure for the molecule eqXeF_4 eq. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
To find the number of valence electron you get in one molecule of xenon oxytetrafluoride add the number of valence electrons of each individual atom that makes up the molecule. Is XeF4 an octahedral. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
How many non bonding electron pairs are there at the Xe atom of the Lewis structure of XeCl 4. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less.
Find octet e- for each atom and add them together. It is a type of noble gas having the chemical equation of. In this tutorial we will learn how to draw lewis structure of XeF4 step by step.
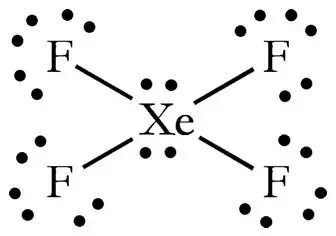
The molecule has octahedral electron geometry and square planar molecular geometry. The main purpose of these Lewis structures is to show that the octet rule is quite important during chemical. In XeF4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom.
What is the lewis structure Vsepr bartleby. Bromine gets 12 electrons in order to make 5 bonds with surrounding atoms. XeF4 Lewis Structure Molecular Geometry Hybridization and MO Diagram Lewis Structure.
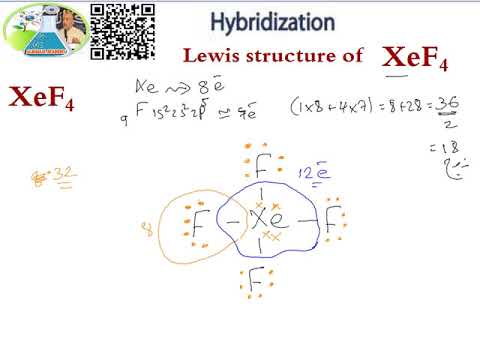
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. The Lewis structure for XeF4 has a total of 36 valence electrons. The Lewis structure for XeF4 has a total of 36 valence electrons.
What is the lewis structure Vsepr Formula and geometric shape of these molecules. Draw and explain the Lewis structure for the molecule XeF4 which does not follow the octet rule. To begin with the Lewis structure of the compound xenon tetrafluoride it is quite essential to know.
The Lewis structure for XeF4 has a total of 36 valence electrons. XeF4 Lewis Structure. These fluorine atoms will then be placed on either side of the central atom.
Each fluorine atom has three lone pairs. XeOF4 you need to start by drawing its Lewis structure. It has two lone pairs of nonbonding electrons on the central atom of Xenon.
It is the worlds first binary compound discovered. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. Draw Lewis-dot structures molecular geometries for XeF4 SF4 SiF4 CF4 and SnF4.
Draw Lewis-dot structure molecular geometry for methane. What hybridization is suggested by the bond length and angles in methane. The geometry of molecules which is also commonly known as molecular structure.
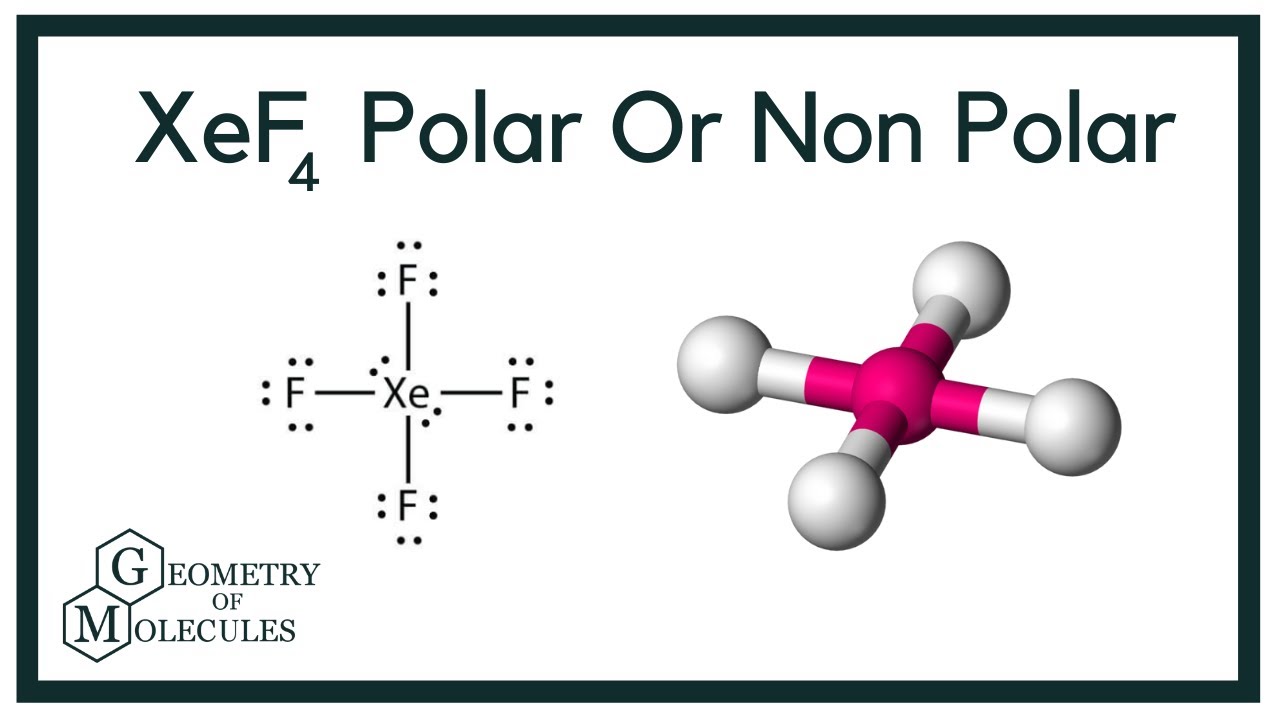
XeF4 is a nonpolar molecule and has sp3d2 hybridization.

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
How To Determined The Lewis Structure For Scl4 Quora

Xef4 Lewis Structure And Molecular Geometry Youtube
Lewis Structure Hybridization Xef4 Youtube

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Xef6 Lewis Structure How To Draw The Lewis Structure For Xef6 Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
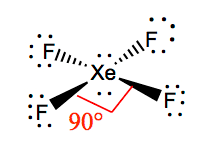
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
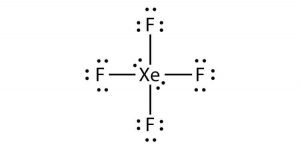
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
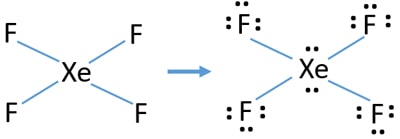
Xef4 Xenon Tetrafluoride Lewis Structure
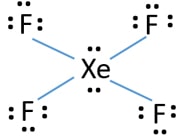
Xef4 Xenon Tetrafluoride Lewis Structure

Xef4 Lewis Structure And Molecular Geometry Youtube
What Is The Vsepr Structure Of Xef4 Quora

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules