Cocl2 Lewis Dot Structure
If you are struggling to do this look at the structural formula and replace the bonds and lone pairs with dots. Lewis Structure is a 2D diagrammatic representation of the arrangement of electrons note.
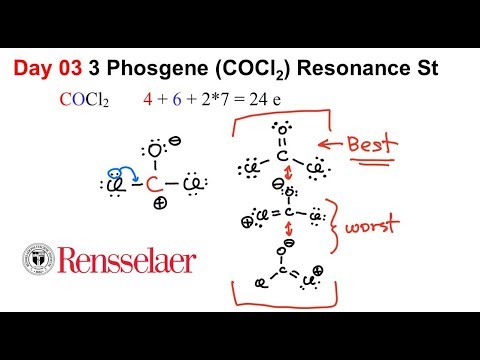
Day03 3 Resonance Structure Of Phosgene Cocl2 Youtube
Therefore the dot resonance structures of COCl 2 Lewis structures of COCl2 are as follows.
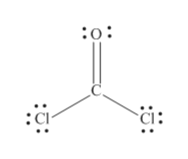
Cocl2 lewis dot structure. In the Lewis structure for COCl 2 there are a total of 24 valence electrons. Cobalt II chloride is an inorganic compound of cobalt and chlorine with the formula CoCl2. It gives us a graphical sketch with electron-dot notations for us to grasp the process in a simple manner.
Both Chlorines have eight valence electrons so their octets are satisfied. If any resonance structures are possible Lewis structures then. Expert Answer 97 111 ratings The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar.
For mathN_2Cl_2math you can look at dinitrogen difluoride as providing your structur. To understand it in detail we have to first get acquainted with the concept of Lewis Structure. 100 muCi in air was delivered through a non-rebreathing system and a tight-fitting face mask for 7-17 minutes.
It is an inorganic compound that comprises Cobalt and Chlorine atoms. CF2Cl2 is a tetrahedron with 4 bonds and no lone pairs. Claims of the formation of tri- and tetrahydrates have not been confirmed.
So thats the Lewis structure. The initial step is to calculate the valence or outermost shell electrons in a molecule of COCl2. CoCl2 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape.
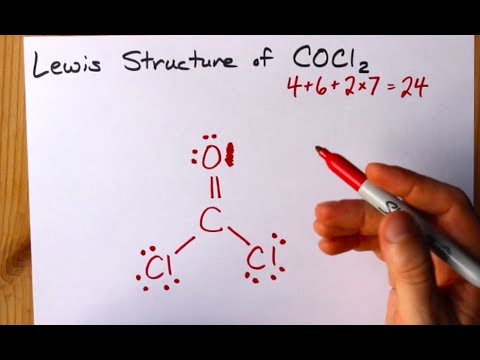
C4O6Cl2x714 Total24 Put carbon in the center. O 4 electrons from lone pairs plus 2 electrons from bonds 6 electrons. It is readily soluble in water alcohol and acetone.
That would be the same as any dot structure. Write these charges next to the atoms in the Lewis structure. The chemical formula CoCl2 represents Cobalt II Chloride.
Moreover there exist many lone pairs which do not alter the molecular geometry but make the molecule polar. Both CCl bonds are polar due to the difference in electronegativity of C and Cl. Youll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom.
I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. Alternatively a dot method can be used to draw the lewis structure of COCl 2. Show the formal charges for all elements in each structure and indicate how you arrived at them.
Cl 6 electrons from lone pairs plus 1 electron from a bond with C 7 electrons. I also go over hybridization shape and bond angles. For the molecule COCl 2 write the possible Lewis Dot structures and indicate the correct one based on formal charge arguments.
And then the Oxygen in the center it also has an octet. Calculate the total valence electrons in COCl 2 molecule. Check me out.
Cannot be accurately described by a Lewis structure consistent with the octet rule. The compound forms several hydrate s CoCl2 n H2O for n 1 2 6 and 9. Lewis dot structure 2 is the more probable most stable since there is no charge separation.
CoCl2 is a crystalline solid that is sky-blue in color. Valence electrons inside a molecule. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule.
C 0 lone pairs plus 4 electrons from bonds 4 electrons. Lewis dot structure of CO Cl 2. It is a sky blue crystalline solid.
What is the Lewis dot structure of COCl2. So we have 2 4 6 8 10 12 14 16 and then back to the center 18 and 20. A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure PhosgeneFor the COCl2 structure use the periodic table to find the total number of.
In the COCl 2 Lewis structure Carbon is less electron electronegative than Oxygen and goes in the center of the Lewis structure note that Hydrogen atoms always go on the outside. So weve used all 20 valence electrons for the OCl2 Lewis structure. Carbonyl fluoride COF2 is a toxic and inflammable compound whose Lewis structure determines the presence of a double bond between the carbon and oxygen atoms and single bonds between the carbon and fluorine atoms.
A step-by-step explanation of how to draw the SOCl2 Lewis Dot Structure Thionyl chlorideFor the SOCl2 structure use the periodic table to find the total n.
How To Calculate The Formal Charge Of Cocl2

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube
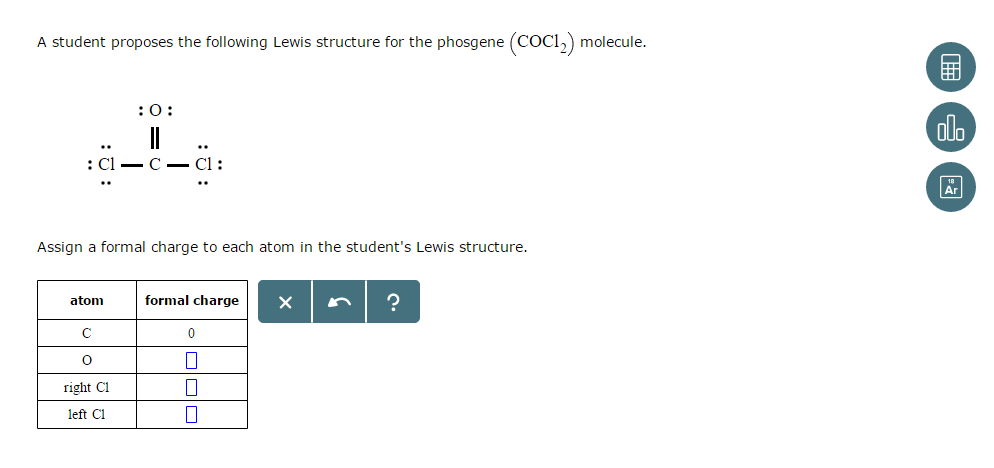
A Student Proposes The Following Lewis Structure For Chegg Com

What Is The Lewis Structure For Cocl2 Study Com
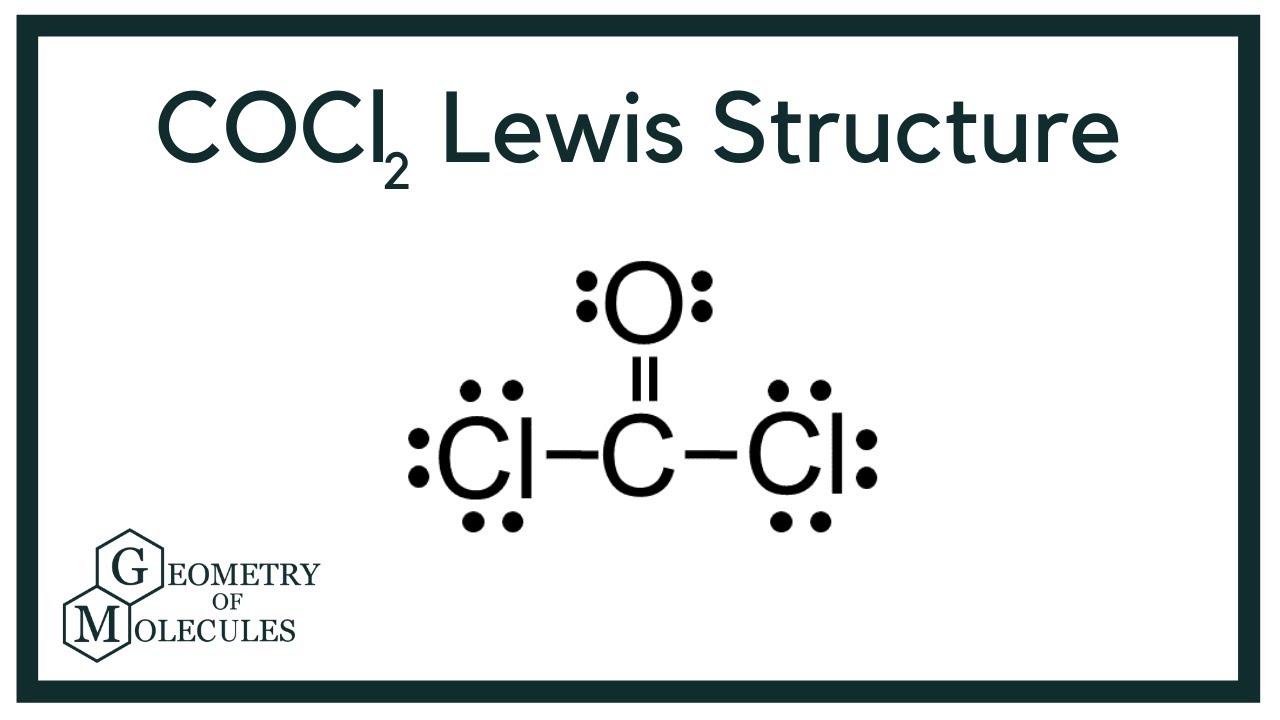
Cocl2 Lewis Structure Phosgene Youtube

How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

The Lewis Structure Of Compound Cocl2 Contain How Many Lonw Pair Of Electrons

Suka Chemistry Which Is The Most Stable Lewis Structure For Cocl2

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Dot And Cross Structure For Cocl2 Phosgene Youtube
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Cl2co Lewis Structure How To Draw The Lewis Structure For Carbonyl Dichloride Youtube

Answered What Is The Molecular Shape Of Coc12 Bartleby