What Is The Molecular Shape Of Scl2
The bond angle of SCl2 is approximately 103 and its bond length is 201 pm. The molecular geometry of Cl2 is linear which is highly symmetrical and in straight lines.

Is Scl2 Polar Or Nonpolar Techiescientist
The electron pair.
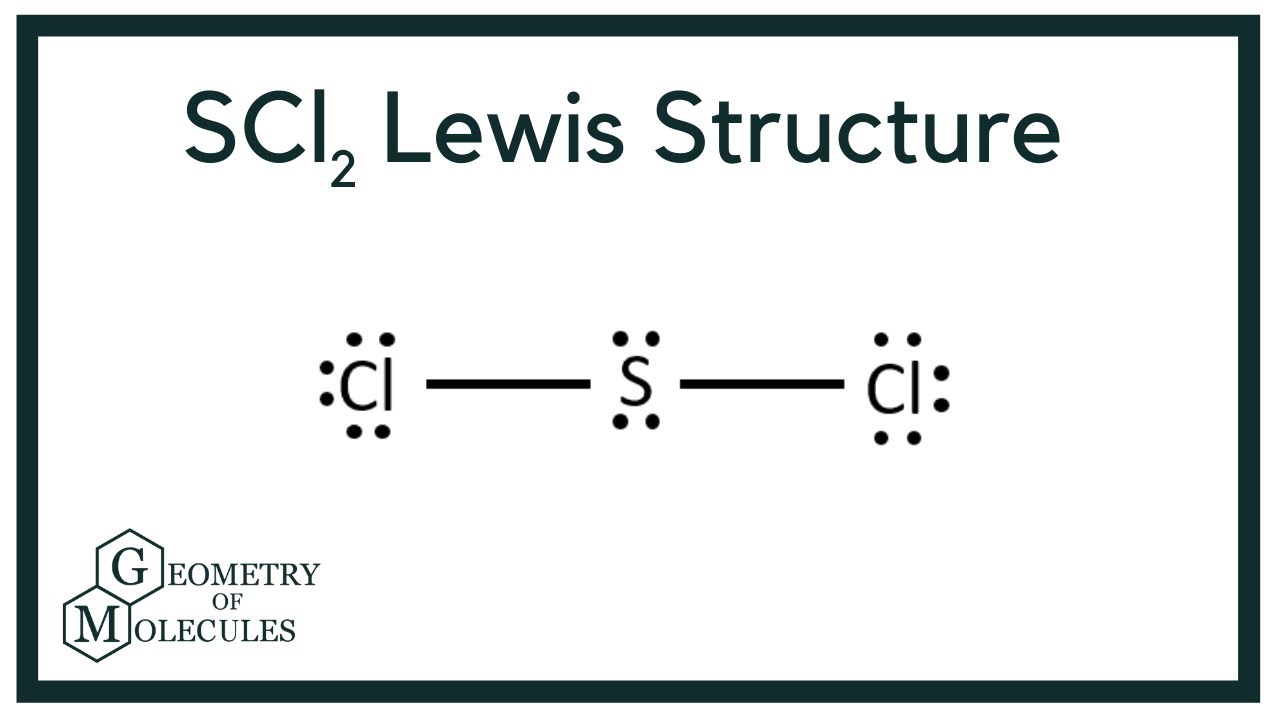
What is the molecular shape of scl2. S which is the molecules central atom has a steric number equal to 4 and a coordination number equal to 2. Based On VSEPR What Is The Molecular Shape Of SCl2 Relative To Sulfur. On the other hand the bond angle of water is 10445 and bond length is 9584 pm.
The molecular geometry of SCl2 is bent which is similar to water but there is a difference between its bond angle and bond length. IodomethaneCH3I has the composition of one carbon one iodine and three hydrogen atoms. SCl2 consists of a single Sulfur atom surrounded by two Chlorine atoms.
Because the core central atom sulfur has two S-Cl bonds with the surrounding two chlorine atoms. To determine the molecular geometry of a molecule one must first determine the electronic geometry by drawing the Lewis structure. 1 See answer Ryther422 is waiting for your help.
Based On VSEPR What Is The Molecular Shape Of SCl2 Relative To Sulfur. Log in Sign up. Drawing and predicting the CH3I molecular geometry is very easy by following the given method.
What is the molecular shape of SCl2. 8 rows SCL2 molecular geometry or shape. Here in this post we described step by step to construct CH3I molecular geometry.
This is in contrast to the electronic geometry which describes the shape of all electron regions. Best Answer 100 7 ratings Previous question Next question. Also the equal number of lone pairs present on each chlorine atom in the Cl2 lewis structure.
A bent B linear C tetrahedral D trigonal pyramidal E not enough information. The bond angle of SCl2 is approx 103º. Hydrogen iodine and carbon come from the1st 17th and 14th.
Notice that SCl2 has a molecualr geometry that is very similar to waters the only differences being. 1 Expert Answer First to determine if it is ionic or covalent molecular look to see if it is a metal bonded to a non metal. Add your answer and earn points.
It is covalent molecular. Is KCl covalent or ionic. Click here to get an answer to your question What is the molecular shape of SCl2.
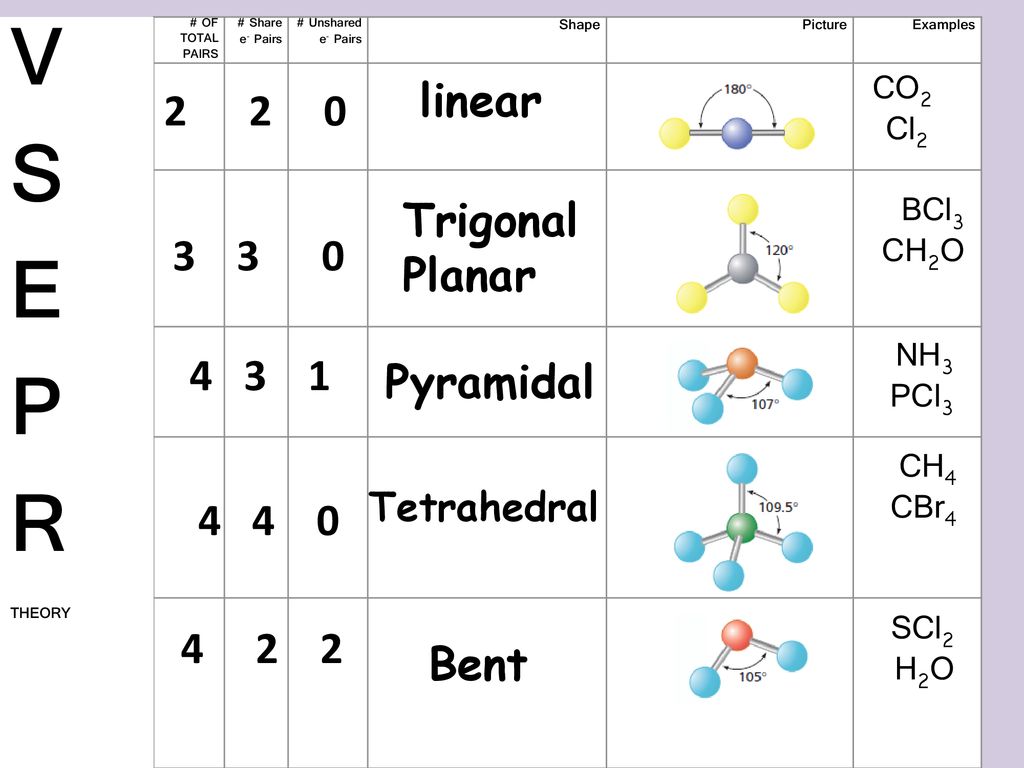
Based on VSEPR what is the molecular shape of SCl2 relative to sulfur. Therefore SCl2 has a Bent molecular geometry and a tetrahedral shape in nature. Electron geometry which is determined by the steric number will be tetrahedral while molecular geometry which is determined by the coordination number will be bent.
Secondly the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result the entire molecule also becomes polar and gives a net dipole moment of 054D. In its most stable state Sulfur acts as the central atom and forms two covalent bonds with the Chlorine atoms. Bent or angular two positions of Tetrahedron occupied by two lone pair of electrons Hence option C is correct.
This problem has been solved. So dipole generated in symmetrical shape can easily be canceled out. Get the detailed answer.
In the same plane the Cl-S-Cl bond forms a 103-degree angle. This is NOT the case for SCl2. What is the molecular geometry of iodomethane.
The shape of some covalent molecule is described in the form of a Lewis structure. The molecular geometry of a molecule describes the three-dimensional shape of just the atoms. SCl2 has a V-shaped bent molecular geometry and water like electron geometry according to the VSEPR theory.
It contains some spatial distribution of shared valence electron pairs in the form of covalent. Free unlimited access for 30 days limited time only. What is the electron pair geometry and Molecular geometry of SCl2.
If so it is ionic. Lets quickly summarize the salient features of SCl2. Ryther422 Ryther422 04222015 Chemistry High School answered What is the molecular shape of SCl2.
What is the molecular geometry of SCl2. Answer verified by Toppr Upvote 2. Is SCl2 a covalent compound.
SCl2 Sulfur dichloride is polar in nature because of bent geometrical shape due to the presence of lone pair present on the sulfur atom.

Arrange The Following Acl8 Species In Order Of Decreasing Cl Clutch Prep
Bond Angle Of Scl2 Lewis Structures

Is Scl2 Polar Or Nonpolar Techiescientist
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Sbr2 Molecular Geometry Shape And Bond Angles Youtube

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep
What Is The Molecular Shape Of Scl2

Scl2 Molecular Geometry Sulfur Dichloride Youtube

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube

Scl2 Lewis Structure Sulfur Dichloride Youtube
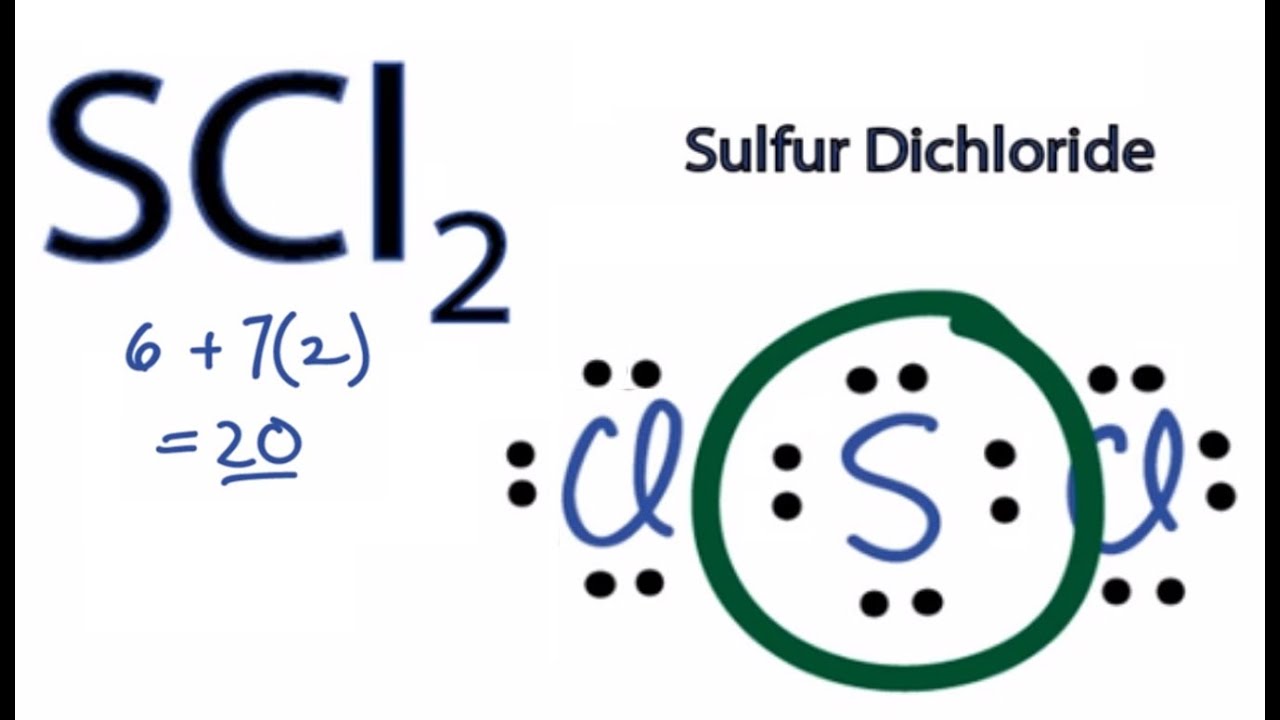
Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

What Is The Lewis Structure For Scl2 Study Com

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

What Is The Electron Pair Geometry And Mol Clutch Prep

The Lewis Diagram For Scl2 The Electron P Clutch Prep

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds

Does Scl2 Have A Dipole Moment Clutch Prep
