What Is The Bond Angle Of Ncl3
This lone-pair of electrons push the N-Cl bond-pair further from the 120 degree angle giving rise to a lower bond angle. This means that the.
What Is The Molecular Geometry Of Ncl3 Quora
The one hour LC50 is 112 ppm with the 95 confidence interval between 107 ppm and 117 ppm.

What is the bond angle of ncl3. So the lone pair is strongly attracted by nitrogen atom in NF3 than NCl3. Smallest bond angle in the following isN C l3 P C l3 S bC l3 AsC l3. What shape is ammonia bond angle and why.
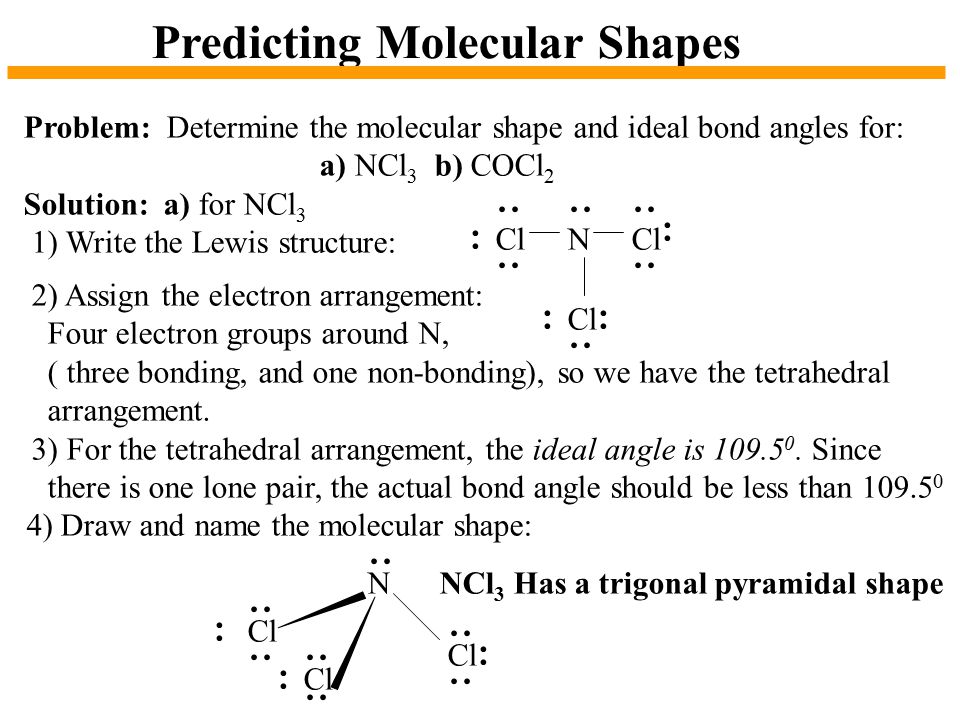
In NCl3 N has one lone-pair of electrons. The enthalpy of formation of NCl 3 is 232 kJ mol -1 a fairly large endothermic value a good sign that a compound is unstable. ½ N 2 g 32 Cl 2 g NCl 3 l.
Trigonal pyrmaidal 107 one lone pair. Acute Exposure The LC50 of nitrogen trichloride NCl3 was determined in rats following a one hour inhalation exposure. This small difference between the predicted angle and the observed angle can be explained by proposing that the unshared pair of electrons on nitrogen repels the adjacent bonding pairs more strongly than the bonding pairs repel each other.
Is NCl3 polar or nonpolar. Smallest bond angle in the following is NCl3 PCl3 SbCl3 AsCl3 A NCl3 B PCl3 C SbCl3 D AsCl3. Experimental evidence of this double bond character.
Tetrahedral no lone pairs 1095. Trigonal pyramidal 107 one lone pair. NCl3 takes this shape due to it following the AX3 E1 format.
As per VSEPR theory the lone pair present on a molecule generates repulsive forces between lone pair and bond pairs. NCl3 has bond angles of 107 degrees whereas BCl3 has bond angles of 120 degrees. Five groups of 10 animals per group were exposed to a concentration range of NCl3 from 58 ppm to 157 ppm.
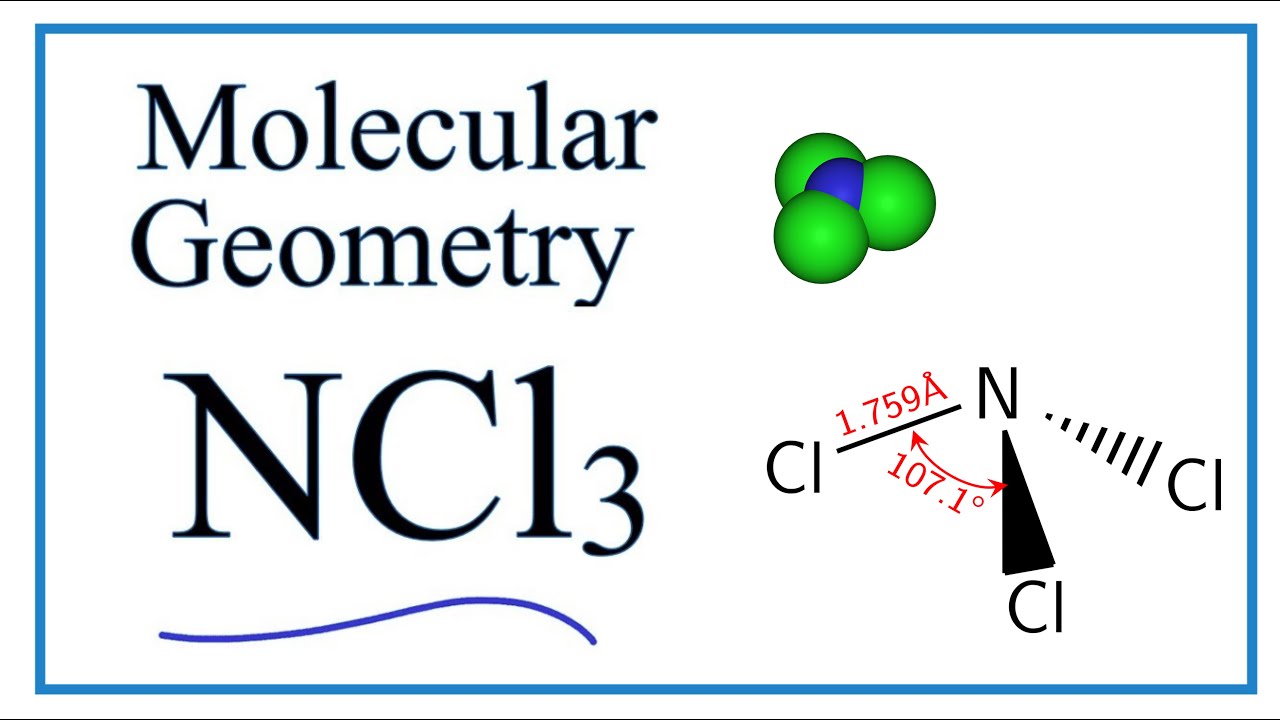
This means that the enthalpy change for the reverse reaction the decomposition of NCl 3 is pretty exothermic. The bond angle of NCl3 is 1071 as it slightly decreases because of the lone pair present on nitrogen that creates repulsion between bond pairs and lone pair hence causes to decrease in angle. Although the bond angle should be 1095 degrees for trigonal pyramidal molecular geometry it decreases to 107 degrees due to the lone pair on the nitrogen atom.
NCl3 By signing up youll get. The observed bond angle is 1073. The ideal bond angle is 1095 but because that lone pair is there all youd have to really say is you would expect the bond angle to be less than 1095.
This gives rise to a stable 120 degree angle between each of the bond-pair electrons. Rank the X-Cl-X bond angles in the compounds below from smallest to largest. Smallest bond angle in the following is.
Here since you have two lone pairs you could say the same exact thing again its electronic geometry is still AX4 ideally it should be 1095 but the lone pairs being there make it less than 1095. Check Answer and Solution for abo. The bond angle of NCl3 is greater than NF3.
This refers to the process. What shape is BCl3 bond angle and. What shape is methane bond angle and why.
As a result the N-Cl bond faces a downward force and the shape of the molecule turns to trigonal pyramidal. The bond angle of Cl-N-Cl is around 1071 degrees and the bond length of N-Cl is 1759 A. Therefore it is trigonal planar.
So the percentage of s-orbital character of the sp3 hybridised orbitals of nitrogen is more in NF3 that in NCl3. This indicates the presence of some partial double bond character which shrinks the bond length. What shape is NCl3 bond angle and why.
NCl3 molecular geometry consists of a trigonal pyramid structure. In ceNCl3 ceN-Cl bond length is 1759 angstroms but ceN-Cl single bond length is 191 angstroms.
Why Does Ncl3 Have A Greater Bond Angle Than Nh3 Quora

Why Does Ncl3 Have A Greater Bond Angle Than Nh3 Quora
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora

Bond Angles Tricks To Compare Bond Angle For Different Molecules Jee Iit Neet Csir Net Youtube
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora
The Bond Angle In Bf3 Is 120 Degrees But In Ncl3 Is 103 Degrees Why Quora
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

Determine The Electron Geometry Eg And M Clutch Prep
Ncl3 Molecule Of The Month April 2017 Jsmol Version

Determine The Electron Geometry Eg And M Clutch Prep
Chapter 10 The Shapes Of Molecules Ppt Video Online Download

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Is Ncl3 Polar Or Nonpolar Techiescientist

Bond Angles In Nh3 And Ncl3 Chemistry Stack Exchange
Ncl3 Molecule Of The Month April 2017 Jsmol Version

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube