Lewis Structure For Hcn With Formal Charges
Find valence e- for all atoms. For the HCN Lewis structure calculate the total number of valence electrons for the HCN molecule.
Two Posssible Lewis Structures For The Molecule Hcn Chegg Com
Hydrogen Cyanide HCN.
Lewis structure for hcn with formal charges. Formal charges are just that - a formality a method of electron book-keeping that is tied into the Lewis system for drawing the structures of organic compounds and ions. It also aids with understanding the bonds formed in the molecule and the electrons not participating in any bond formation. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
To start with making the Lewis Structure of HCN we will first. Spts United States. Once you get the total number of valence electrons you can make a Lewis dot structure of HCN.
All possible resonance structures for OCN. Please provide as many details about your writing struggle as possible. HNO 3 Nitric acid Lewis Structure HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
Chem 1090 Lewis 6a Chem 1090 Lewis 6a About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How. So how do you decide which is the correct Lewis Structure. For HCN above is the Lewis Structure.
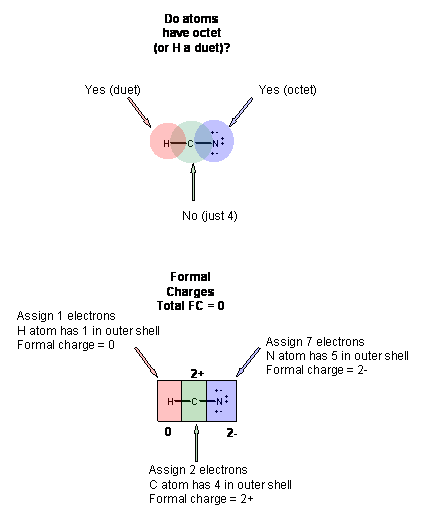
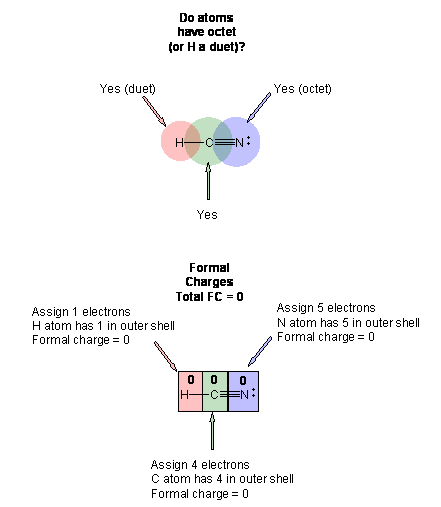
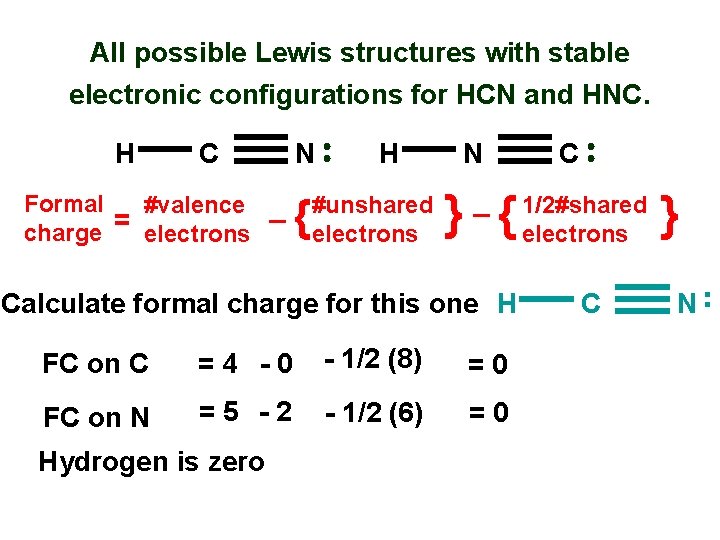
The carbon dioxide Lewis structure and formal charge. What we see on the Lewis dot structure on the left H-Cequiv N is that the star is around hydrogen the diamonds are around carbon and the circles are around Nitrogen and so as in the above equation the formal charge of all species is zero. The best Lewis dot structure has the most atoms with zero formal charge or the closest to zero.
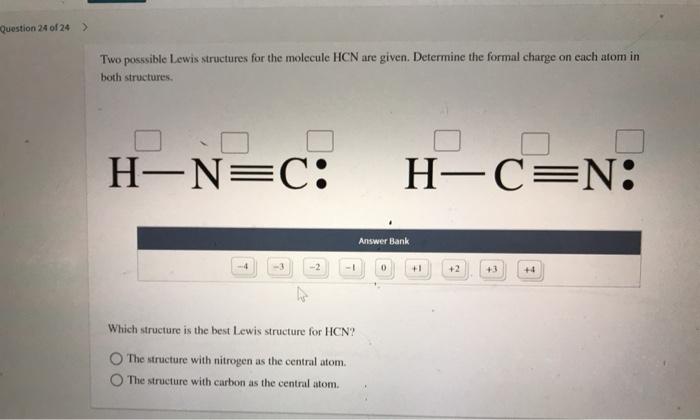
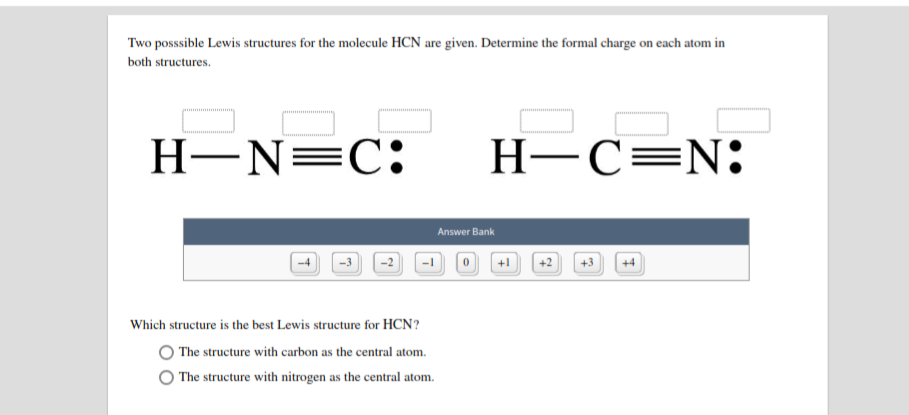
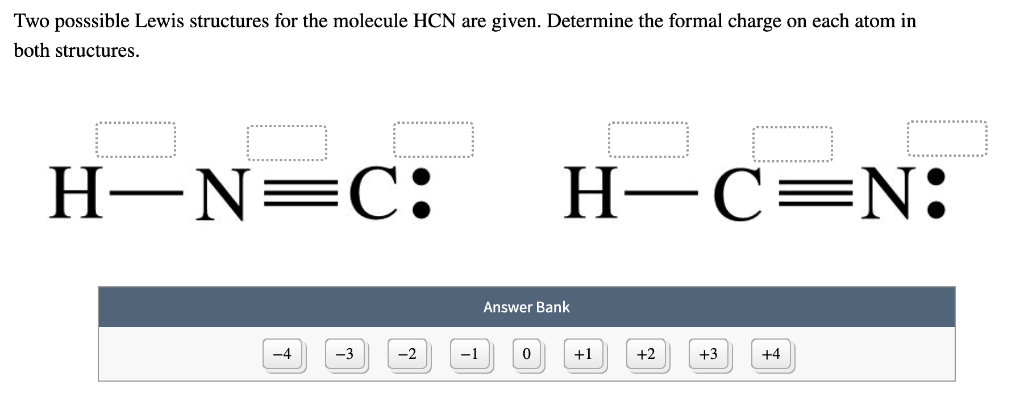
Connect with a professional writer in 5 simple steps. The structure with carbon as the central atom. Two posssible Lewis structures for the molecule HCN are given.
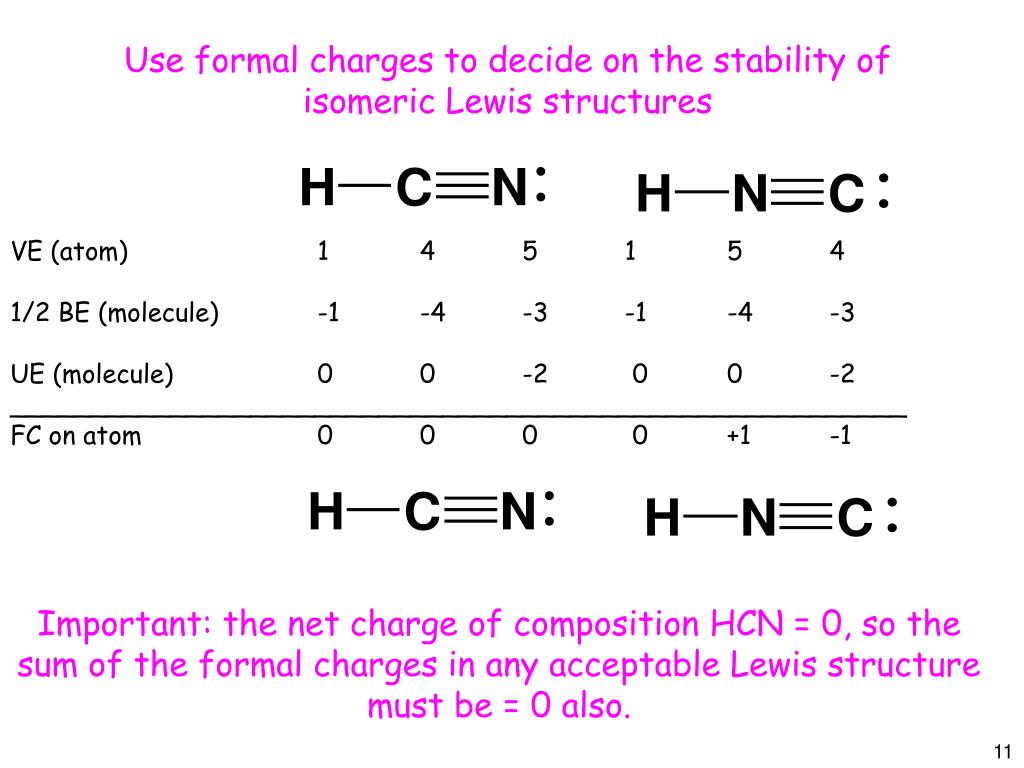
But there are molecules where the Lewis structure can be written in different ways still satisfying the Octet rule. So the formal charges areN 5-50 C 4-4 0 H 1-1 0Formal charge is different than actual charge and above is a covalent compound so there is no actual charge on the atoms they are not existing as cation or anion. In HCN the valence e- are.
What is the lewis structure for hcn. 5pts Lewis Structure Formal Charge of H Formal Charge of C Formal Charge of N 7. To draw the Lewis structure for HCN we will first calculate the total number of valence electrons.
If there is a compound that can have more than one Lewis structure while satisfying the Octet rule the correct Lewis Structure would be the one with least amount of formal charge. Later we will see how the concept of formal charge can help us to visualize how organic molecules react. The structure with nitrogen as the central atom.
So I introduce the term formal charge which is different than the actual charge. If you look on the periodic table you will notice that H has one valence electron C has 4 and N has 5. Lewis structure of nitric acid.
Circle the most stable resonance structure. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. H1 C4 N5 Add the total number of e- 145 10.
But in the second structure H-Nequiv C we see that hydrogens star is still around hydrogen and so it formal charge is still zero but one of Nitrogens. Formal charges can help identify the more likely of two isomers that is two different structures made from the same set of atoms like HCN and HNC. Determine the formal charge on each atom in both structures Answer Bank -4-32 Which structure is the best Lewis structure for HCN.
Write the Lewis Structure for HCN and calculate the formal charge for each atom of HCN. If you sum all these valence electrons you will get 10. In order to calculate the formal charges for HCN well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec.
Academic level of your paper Type of Paper. After determining how many valence electrons there are. 1 C4 N5 The lone pair is assigned to N there are 6 e-in a triple.

Hcn Lewis Structure Draw Correctly Step By Step Lewisstr
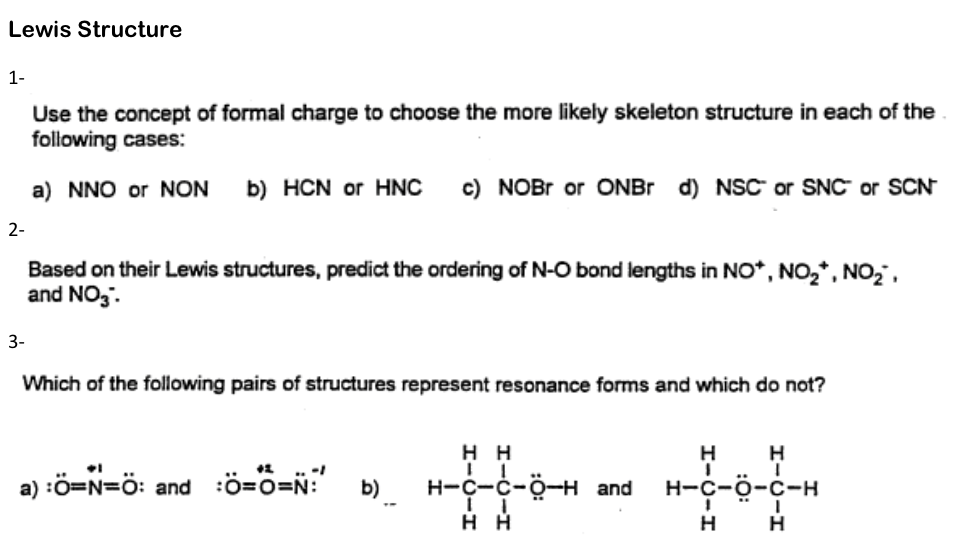
Lewis Structure 1 Use The Concept Of Formal Charge Chegg Com
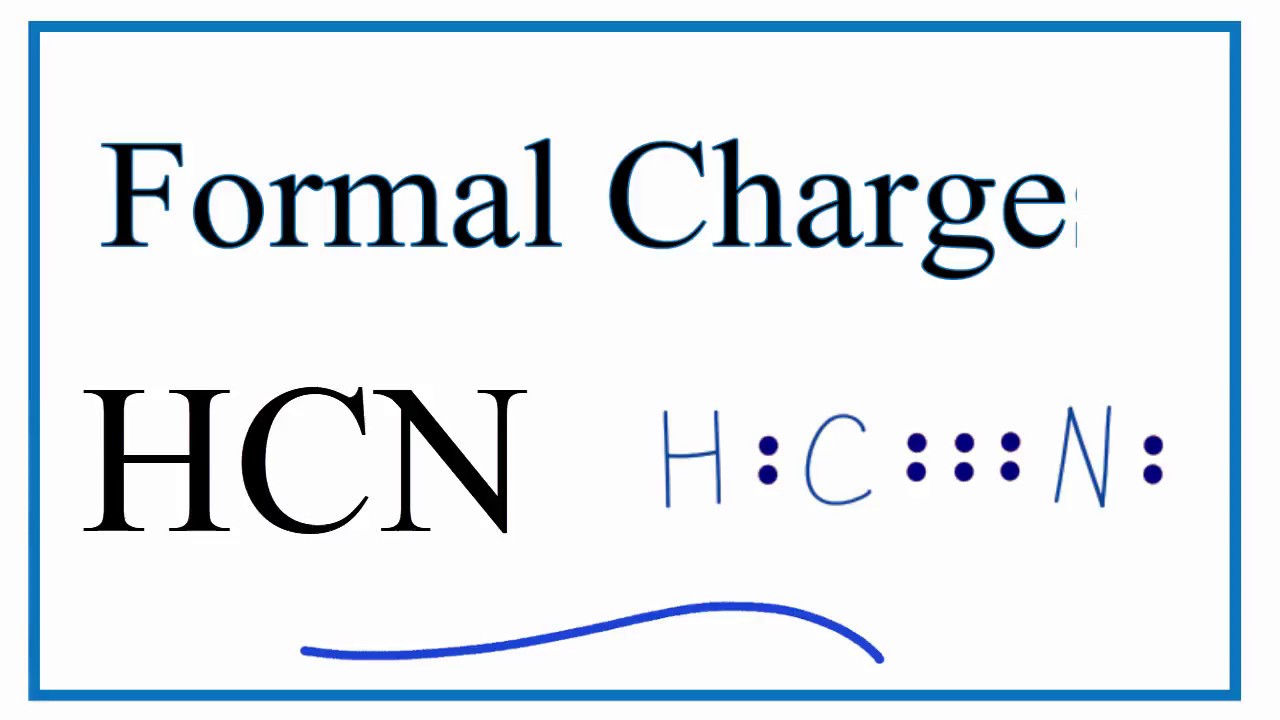
How To Calculate The Formal Charges For Hcn Acetonitrile Youtube

How To Draw The Lewis Dot Structure For Hnc Hydrogen Isocyanide Youtube
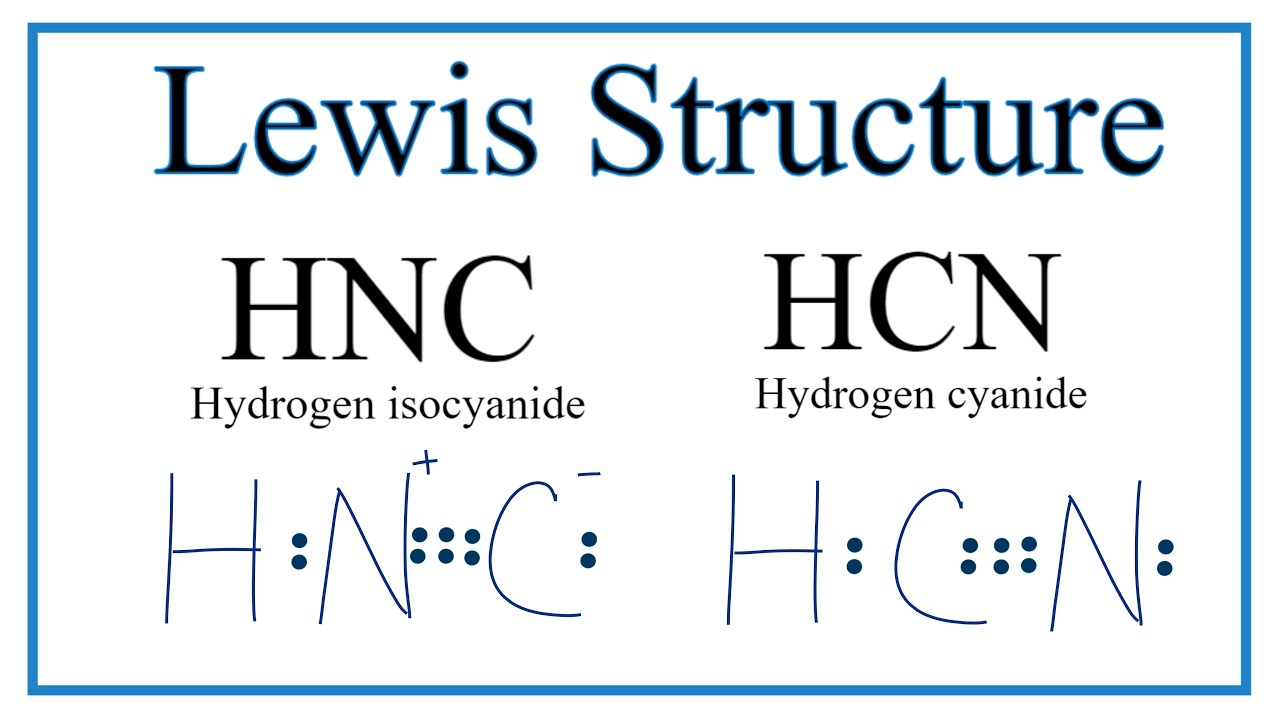
How To Draw The Lewis Dot Structure For Hnc Hydrogen Isocyanide Youtube

How To Calculate The Formal Charges For Hcn Acetonitrile Youtube
Question 24 Of 24 Two Possible Lewis Structures For Chegg Com

Bonding General Concepts Shows How Valence Electrons Are Arranged Among Atoms In A Molecule Reflects Central Idea That Stability Of A Compound Relates Ppt Download

Draw The Lewis Dot Structure Of Hydrogen Cyanide Hcn Molecule

Two Posssible Lewis Structures For The Molecule Hcn Chegg Com

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube
Solved Ch1 What Is The Correct Lewis Structure For Hydrocyanic Acid Hcn Including The Formal Charges If An H C N Hc N H C N H C N H Course Hero
Whats Coming Up Oct 25 Oct 27 Oct
Two Posssible Lewis Structures For The Molecule Hcn Chegg Com
Ppt Lewis Structures And The Geometry Of Molecules With A Central Atom Powerpoint Presentation Id 6380170

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube