The Lewis Structure Of Pf5 Is Given Below. What Is The Hybridization Of The Fluorine Atom
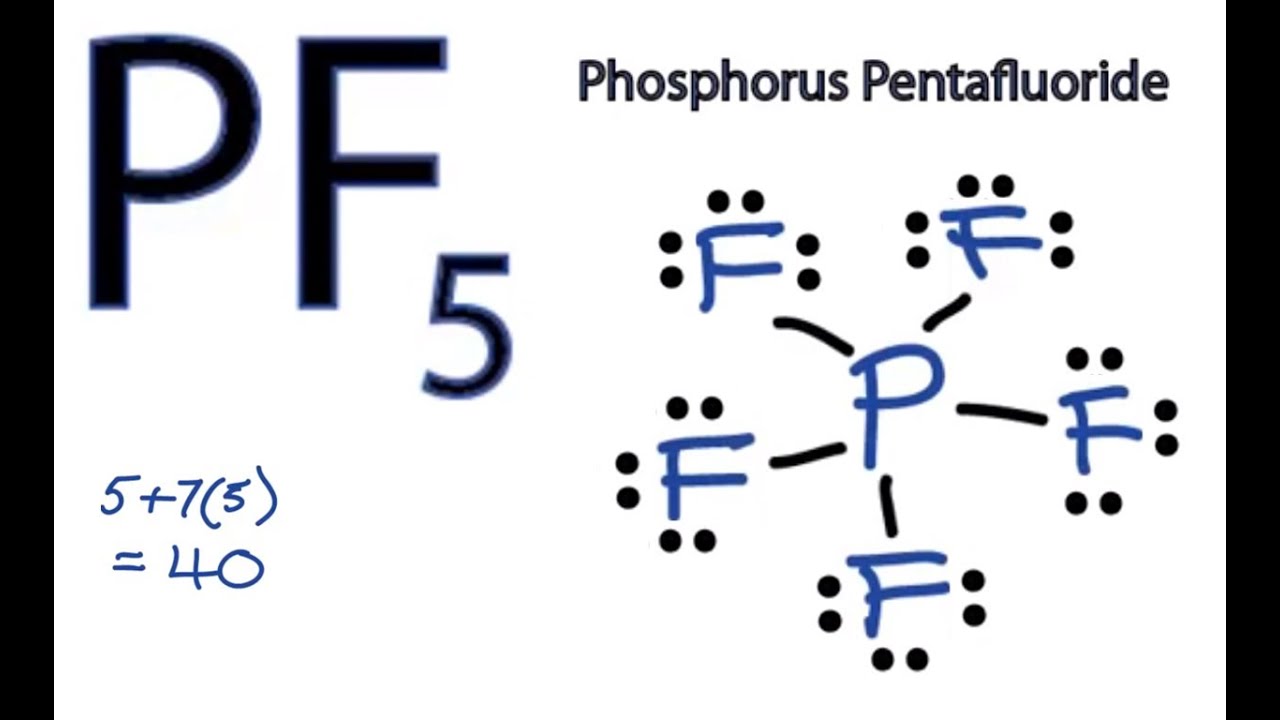
There are no lone pairs in the Lewis Structure of PF 5 and there are five single bonds between Phosphorus and Fluorine atoms. The hybridization is sp.

Sf6 Lewis Structure How To Draw The Lewis Structure For Sf6 Youtube
Calculate the formal charge for P in PF5.
The lewis structure of pf5 is given below. what is the hybridization of the fluorine atom. And we take the oxidation state of halogens as 1. Name of molecule Phosphorus Pentafluoride PF5 Bond Angles. The remaining electrons in the atoms.
So for one F electron is 1 and for F 5 the no. The phosphorus element inside has ten valence electrons. Pe Sp2 Hybrid Spºd Hybrid Sp3 Hybrid Sp Hybrid Spd.
How many valence electrons are in the molecule PF5. Lone pairs are found in one of the hybrid orbitals. Show transcribed image text.
1 The given molecule is As we know that tellurium has 6 valence electrons and chlorine has 7 valence electron. The central phosphorus atom has eqsp3d eq atomic orbital hybridization which accounts for its ability to form five single bonds to the fluorines. What Is The Hybridization On The Fluorine Atom.
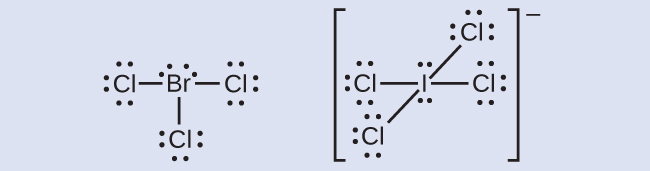
Five valence electrons of bromine will be used to form sigma bonds with 5 F atoms. All five fluoride elements are attracted to the phosphorus element in the center and have eight electrons in their valence shells. Do you think we have missed something about PF5 topic.
The Lewis structure of PF5 is given below. It means the hybridization of ClF3 is Sp³d. Using VSEPR THEORY explain why 1BF4- is a tetrahedral molecule 2SF3 is a Trigonal pyramidal molecule 3ICI4- IS A SQUARE PLANER molecule 4IF5 IS A SQUARE pyramidal molecule 5PF5 IS A TRIGNAL BIPYRAMIDAl molecule help Dr bob222.
Type out your calculation. Is the octet rule obeyed for every atom in the structure. With this atom a single bond is made with the neighboring Bromine atoms which means 2 electrons are shared between these atoms.
The Lewis Structure Of PF5 Is Given Below. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. Here Phosphorous is the central atom.
Phosphorus pentafluoride is a trigonal bipyramidal molecule. Since each bond corresponds to a shared pair of electrons the Lewis structure is. PF 5 Hybridization The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3 but when it is in an excited state the electrons from 3s orbital get unpaired.
Of electrons is 5. In BrF 5 one 4s three 4p and two 4d orbitals take part in hybridization. It consists of PF5 molecules in which each fluorine atom is bonded to the phosphorus atom.
By in situ hybridization the CXCL4 gene is mapped to chromosome 4q12-q21. Calculate the formal charge for F in PF5. Note that all F atoms have the same formal charge.
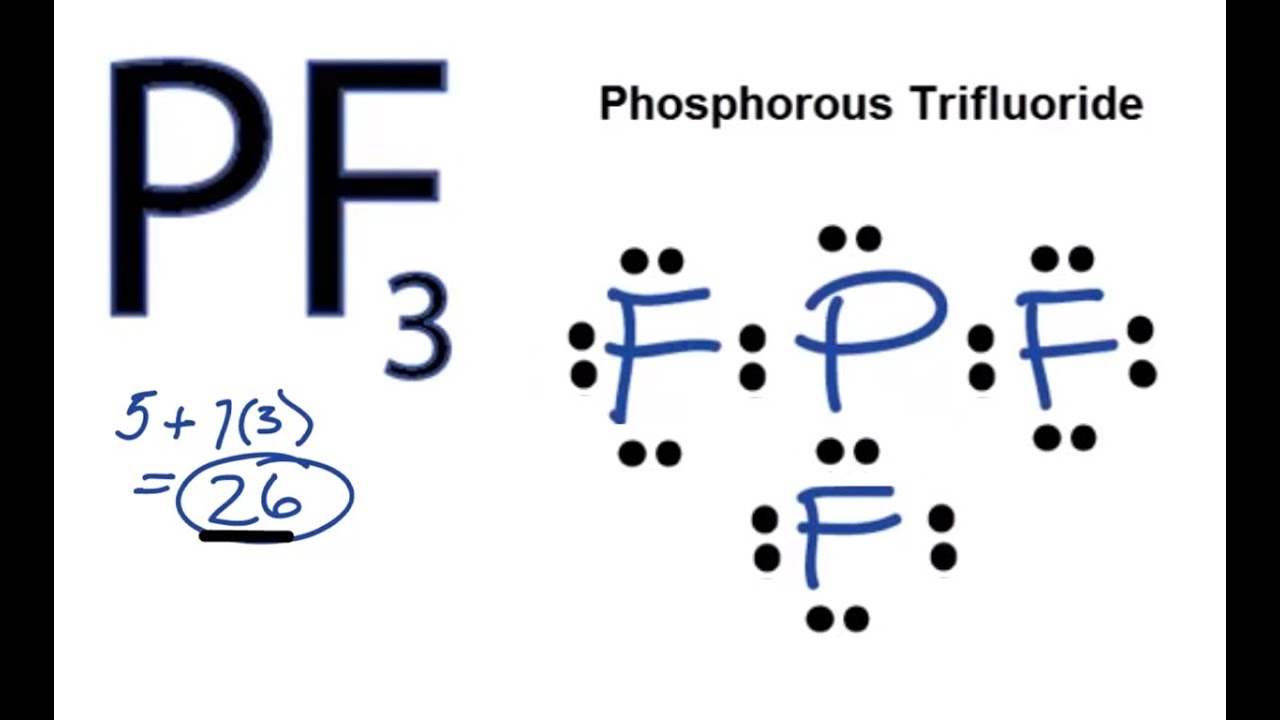
What are New Englanders called. Phosphorous has 3 unpaired electrons available for bonding which is not sufficient to form bonds with five fluorine atoms. 90 and 120 Molecular Geometry of PF5.
It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. In PF5 Phosphorous has 15 electrons and Fluorine has 9 electrons. In P F 5 the valence electrons of phosphorus are 5.
To draw PF5 Lewis structure watch the above video or follow the given instructions. Expert Answer 100 1 rating Previous question Next question Transcribed Image Text from this Question. Let us know in the comments section below we will add them.
Has sp d hybridisation S4. Important Points To Remember. The difference between the number of valence electrons in a free atom and the number of electrons assigned to the atom in a Lewis structure.
Type out your calculation. The compound phosphorous pentafluoride has one phosphorous atom and five fluorine atoms. Look at the lewis structure of ClF3 three fluorine atoms attached to the central atomChlorine and two lone pairs present on the central atom.
The length of an axial PF bond is distinct from the equatorial PF bond in the solid phase but not the liquid or. In every molecule there is one central atom from which other atoms are bonded to. What is the hybridization of PF5.
Examples of molecules with more than an octet of electrons are phosphorus pentafluoride PF5 and sulfur hexafluoride SF6. In order to determine the hybridization of the central phosphorus atom in phosphorus pentafluoride PF5 you must first draw. PF4 has sp hybridisation C TFTT D FFFT 34.
Explain why or why not. Thus it has two distinct types of PF bonds axial and equatorial. Therefore one electron from 3s orbital jumps to 3d level and the orbitals hybridize to form hybrid orbitals.
The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. PBr5 Lewis Structure. The Lewis structure is shown below.
In this type of hybridization one- s and two P-orbitals of the valence shell of carbon atom take part in hybridization go give three new sp 2 hybrid orbitals. This problem has been solved. The hybridization of this compound is sp3d s p 3 d.
The hybridization of P F 5 is sp3d. The total electrons in this compound are calculated by. There are no lone pairs in the Lewis Structure of PF5 and there are five single bonds between Phosphorus and Fluorine atomsPF5 Lewis structure Molecular Geometry Bond angle and Shape.
Lewis Dot Structure for PF5. These sp 2 hybrid orbitals lie in a plane and are directed towards the corners of an equilateral triangle with a carbon atom in the centre. So H 3 2 5 is the hybridization number of ClF3.
The hybridization and are and. The hybridization of carbon atoms in C2 C3 single bond of HC C CH. Phosphorus Pentafluoride PF5 have zero dipole moment.
The central atom bromine forms 5 sigma bonds with fluorine atoms. Use VSEPR theory or AXN method to determine ClF3 molecularelectron geometry. Therefore from phosphorus and fluorine.
The molecule will consist of one lone pair. Phosphorus pentafluoride is a gas at room temperature. Below is the explanation of how the Lewis Structure of PBr5 is made.
Explain why the Lewis. The PF5 has sp3 hybridization and its electronic geometry is the plane triangle.
Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube

P4 Lewis Structure Tetraphosphorus In 2021 Molecules Lewis Electrons

Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

H3po4 Lewis Structure How To Draw The Lewis Structure For H3po4 Youtube
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

Bf4 Lewis Structure How To Draw The Lewis Structure For Bf4 Youtube
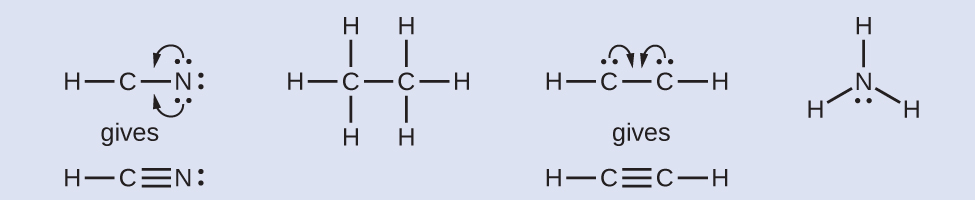
7 3 Lewis Symbols And Structures Chemistry
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
Xef2 Lewis Structure Polarity Hybridization And Shape

Clf Lewis Structure How To Draw The Lewis Structure For Clf Chlorine Monoluoride Youtube
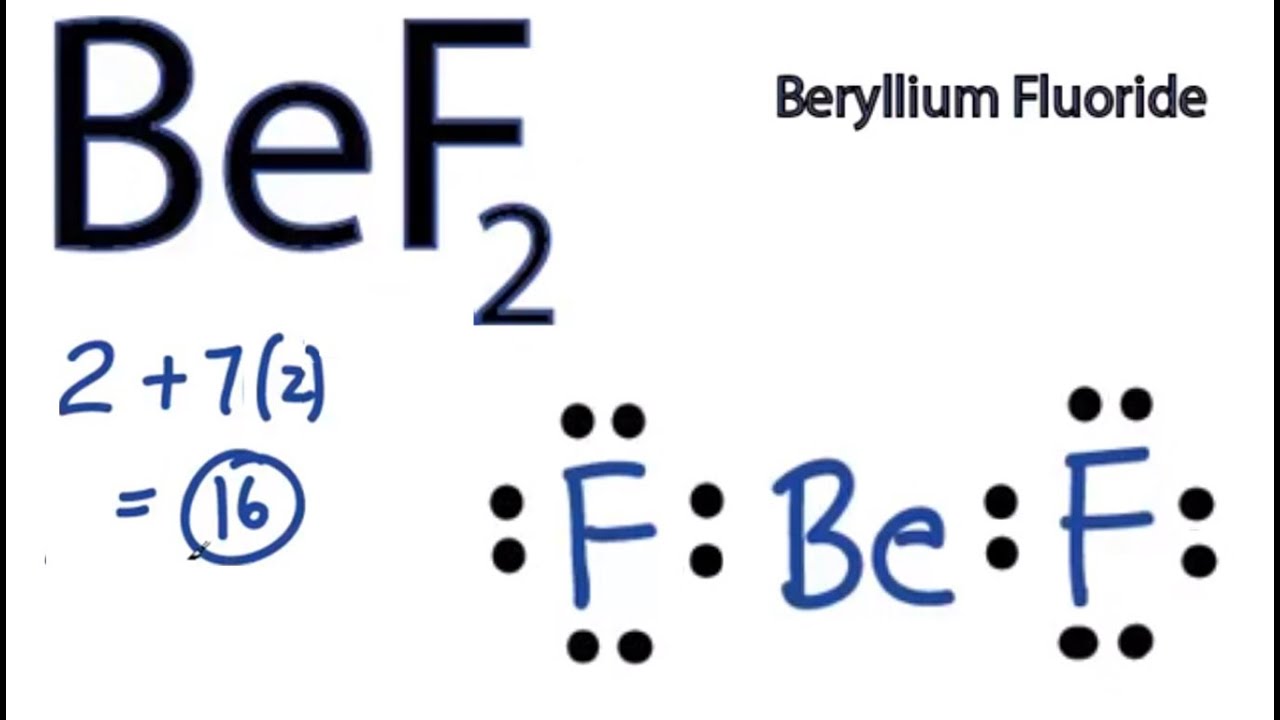
Bef2 Lewis Structure How To Draw The Lewis Structure For Bef2 Youtube
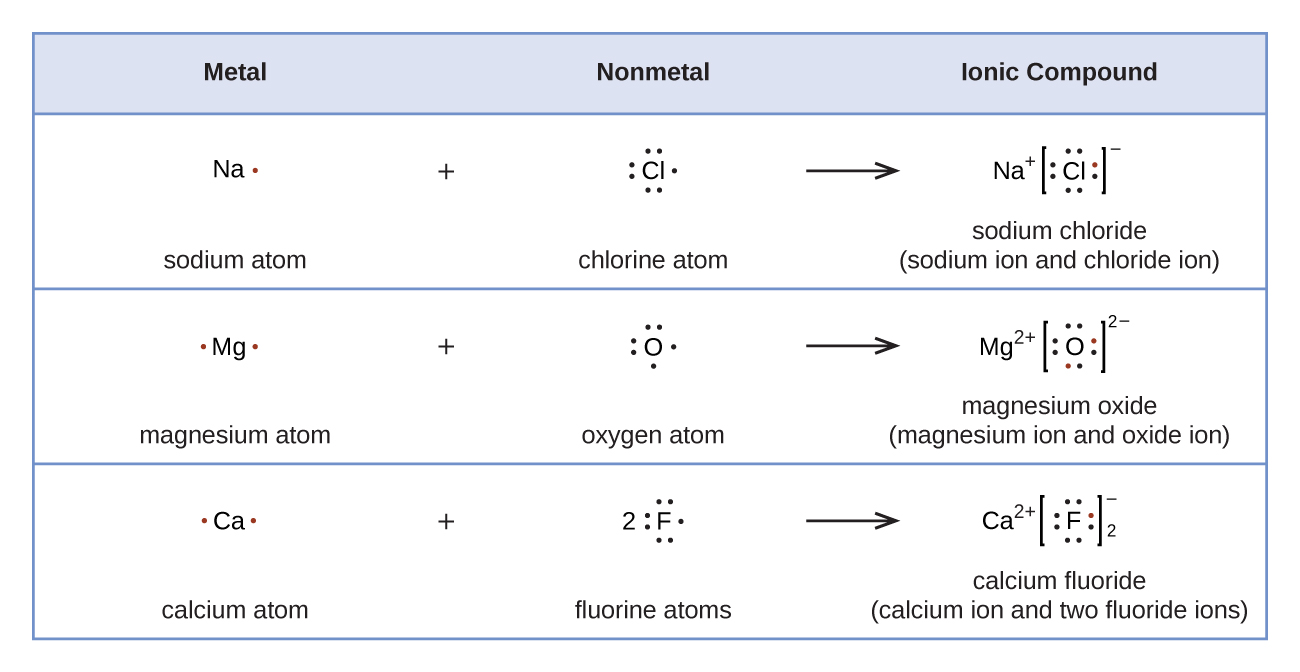
7 3 Lewis Symbols And Structures Chemistry

Icl4 Lewis Structure How To Draw The Lewis Structure For Icl4 Youtube
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

7 3 Lewis Symbols And Structures Chemistry
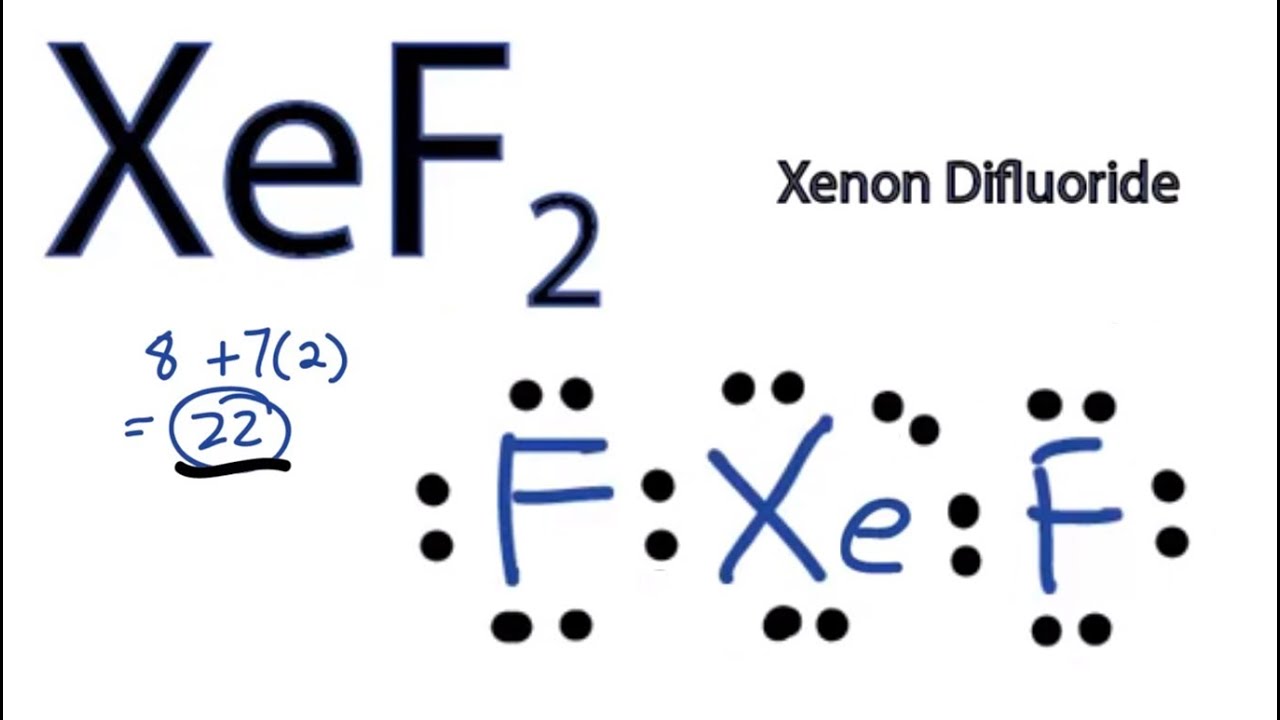
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
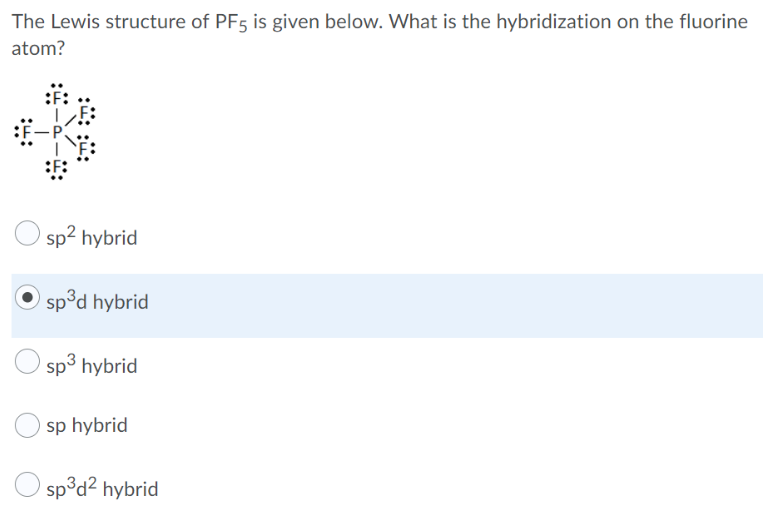
The Lewis Structure Of Pf5 Is Given Below What Is Chegg Com