Draw The Lewis Dot Structure For Co2
Draw The Lewis Electron Dot Structure For Co2 co2 lewis structure dot dioxide carbon electron diagram pairs resonance electrons structures o2 bonds many drawn chemistry draw covalent lone dioxide lewis carbon co2 structure molecular dot draw structures chemical chemistry monoxide co2 lewis structure carbon dioxide draw centre co2 lewis structure dot dioxide carbon electron. In the Earths atomsphere it is considered a greenhouse gas.

Co2 Lewis Structure Carbon Dioxide Youtube
Structure of Carbon Monoxide Carbon Dioxide Oxides of Silicon.

Draw the lewis dot structure for co2. Here are the steps that I follow when drawing a Lewis structure. These valence electrons are represented by drawing dots around the individual atoms hence the Lewis dot structure. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structure.
The reason for drawingcreating a Lewis dot structure is that it helps one predict the kinds of bonds as well as a number of. The hybridization found in eqrmCrmO_rm2 eq molecules is sp hybridization. Drawing CO2 Lewis Structure is very easy to by using the following method.
PDF Oxy-Fuel Combustion for Power Generation and Carbon Dioxide CO2 Capture Woodhead Publishing. In the CO 2 Lewis structure carbon is the least electronegative element. 1969 CO2 Carbon Dioxide Chemistry Physics Science Film.
Total valence electron available for drawing the CO2 lewis dot structure 4 26 16 valence electrons 2. To know the lewis structure of CO2 one should first understand what precisely the Lewis structure is. For the AsO4 x molecule how many equal-energy resonance structures can you.
So total valence electrons are 16. Count the valence electrons in your trial structure. Therefore it is put in the center of the dot structure.
So put the Carbon in the middle and then set the oxygen either side of that. Draw The Lewis Dot Structure For Co2 MakeTheBrainHappy. CO2 Lewis structure So CO2 4 62 16.
In order to complete the octets for all of the atoms in the structure you will need to form two double bonds. Punjab Group Of Colleges. Lewis structure for c2h2 Lewis structure is a theory that helps in understanding the structure of a given.
Drawing lines represent the bonds formed in the. You follow a sequence of steps. I also go over hybridization shape and bond angles.
That will normally be the least electronegative atom C. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. This is the Lewis Dot Structure for CO2.
Find the least electronegative atom and placed it at center. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons.
You could alternatively also draw the structure by including two dots for every bond. Draw a trial structure by putting electron pairs around every atom until each gets an octet. People Also Asked What is the lewis dot structure for co2.
I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. The carbon dioxide chemical formula is CO2. That would mean that you would have a total of eight dots around the carbon thereby filling its octet.
Decide which is the central atom in the structure. The Lewis Dot Structure for CO2 CO2 Lewis Structure How to Draw the Dot Structure for 38 Lewis Structure For Co2 PNG Bepe Enthusiastic Draw the electron dot structure of carbon dioxide co2. Carbon is the least electronegative that means it stays at the center.
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure Carbon dioxideFor the CO2 structure use the periodic table to find the total number. The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. So the Lewis dot structure for the carbon dioxide molecule will be like.
Lewis structure for C2H2O. The Lewis structure for CO2 has a total of 16 valence electrons. Draw Lewis structures to figure this one out a 2 b 0 c -1 d -2 e -3.
In order to complete the octets for all of the atoms in the structure. The octets of both of the oxygen atoms are also satisfied since the oxygens have a total of eight electrons around them thereby filling the valence shell. CHEMISTRY What is the Lewis structure of c2h2o.
Also know how many total valence electrons are used in drawing the Lewis structure of carbon dioxide. For the CO 2 Lewis structure there are a total of 16 valence electrons available. How to draw single bonds using dots to.
Here in this post we described step by.

Lewis Dot Structure Easy Hard Science

Co2 Lewis Structure Easy Hard Science

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Draw The Electron Dot Structure Of Carbon Dioxide Co2 Brainly In

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
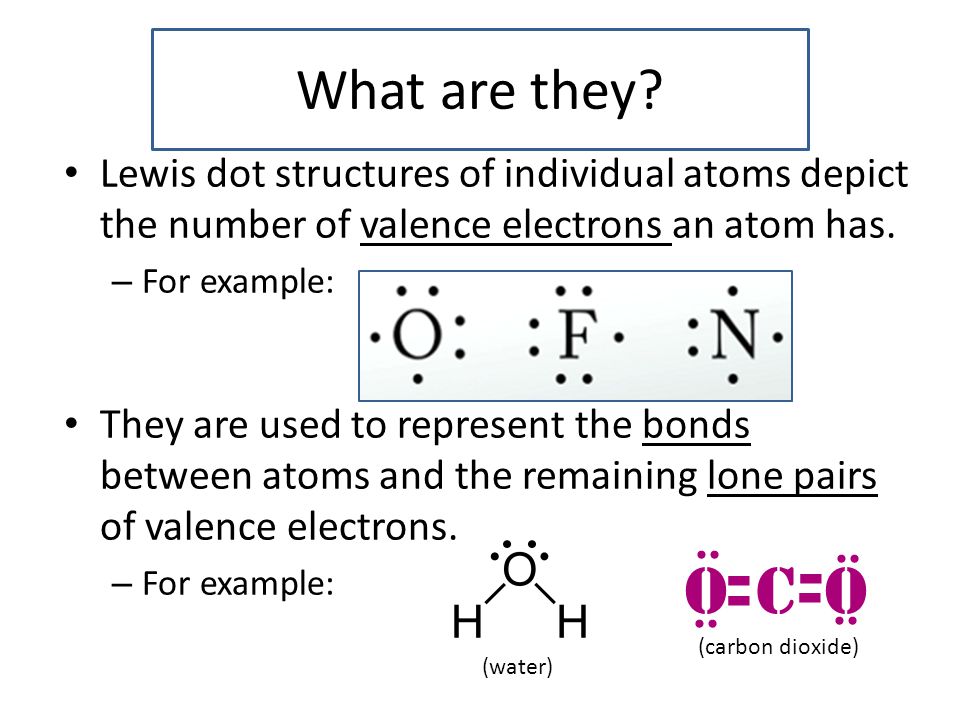
Lewis Dot Structures Ppt Video Online Download

Lewis Electron Dot Structures Detailed Explanation With Examples Videos
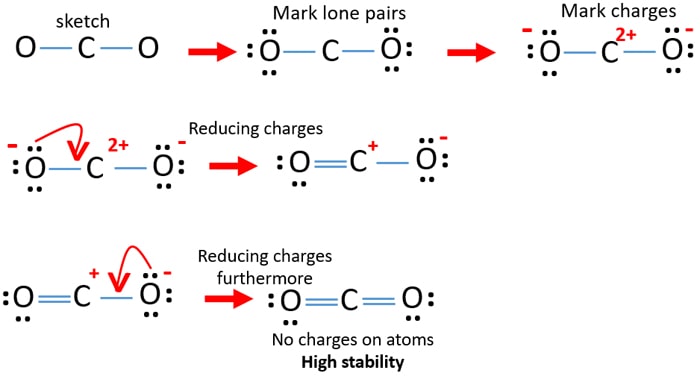
Co2 Carbon Dioxide Lewis Structure And Shape
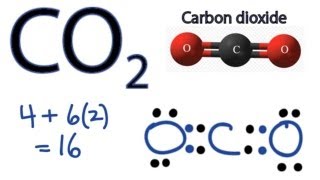
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
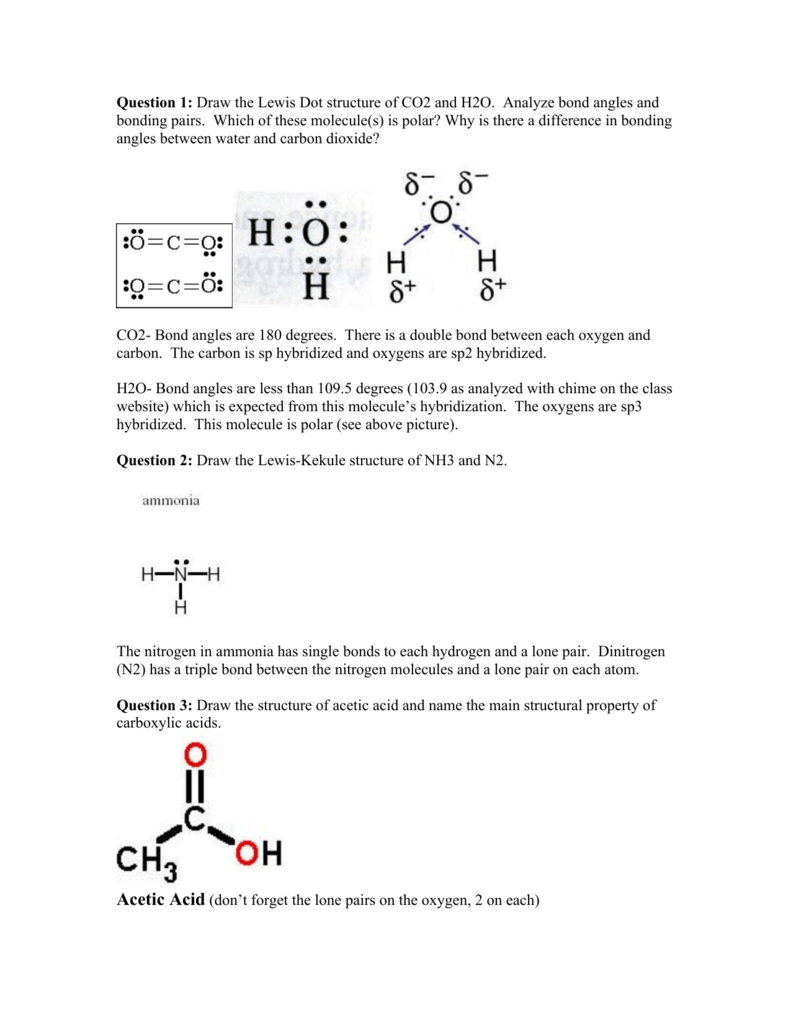
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Co2 Lewis Structure Easy Hard Science
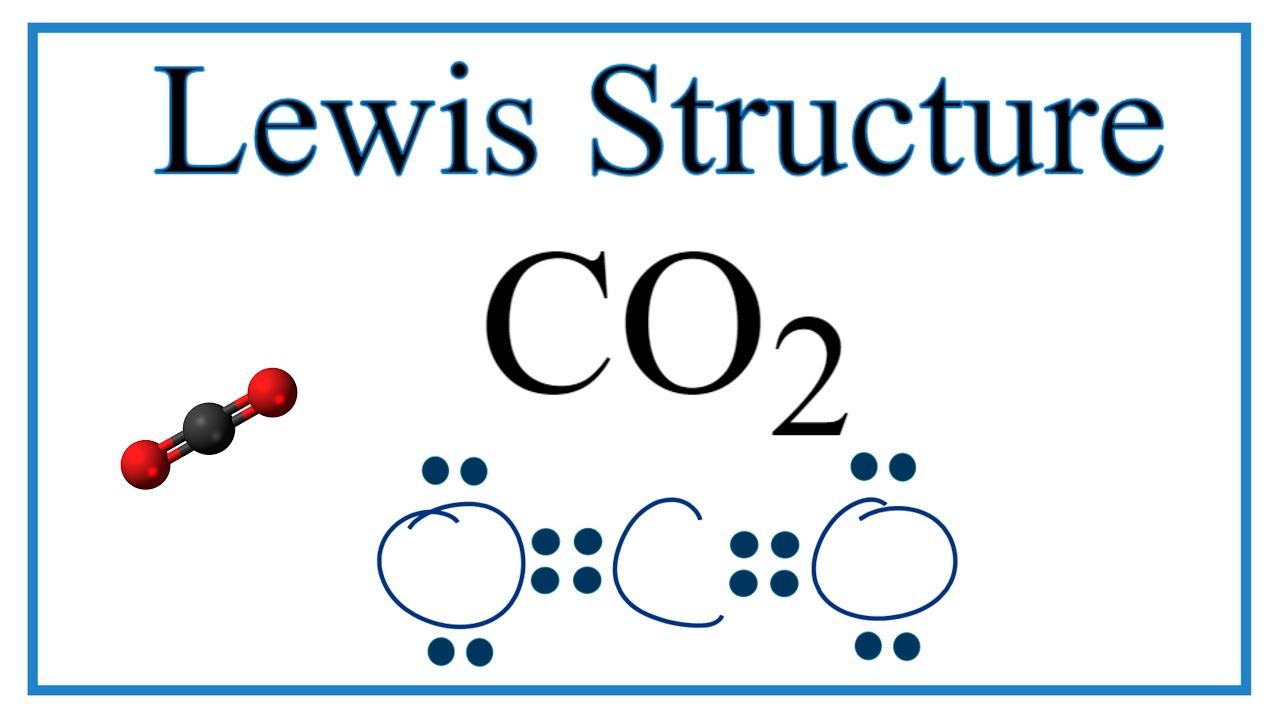
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube

Makethebrainhappy The Lewis Dot Structure For Co2

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Draw The Electron Dot Structure Of Co2 Ch4 S8 Brainly In
Co2 Lewis Structure Molecular Geometry And Hybridization

Makethebrainhappy The Lewis Dot Structure For Co2
