H3o+ Valence Electrons
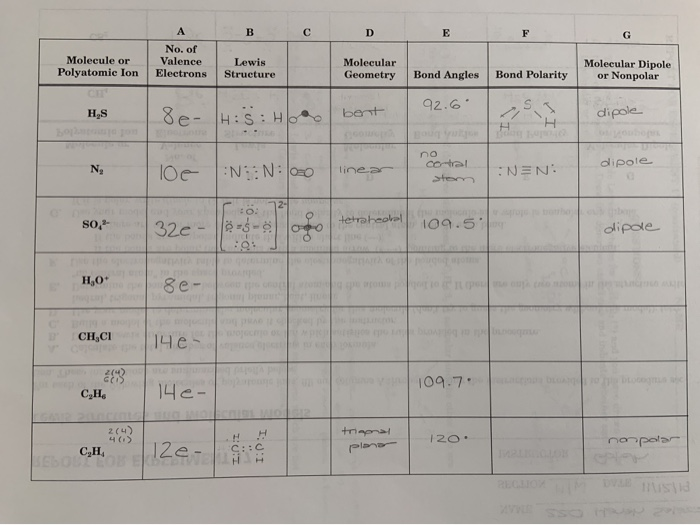
Lewis Structure electron groups electron group geometry of bonded atoms molecular shape Resonance structures if any Polar or nonpolar CO32- SO2 NO2 PF3 SiL Sila Crude Sketch Calculations of valence electrons of bonds etc. 3 hydrogen atoms are bonded to oxygen so the number of the monovalent atoms M 3 As this is a cationic molecule thus C 1.

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
From these three valence electrons of oxygen atom two electrons will be shown as a pair of electrons on oxygen atom but a single electron can not be shown.

H3o+ valence electrons. Note that the sign in the Lewis structure for H3O means that we have lost a valence electron. The Lewis structure for PH3 is similar the the structure for NH3 since both P and N are in the same group on the Periodic table. You can figure out the number of valance electrons for any main.
The valence of the carbon 4 hydrogen 1 and oxygen 6. The PH3 Lewis structure has 8 valence electrons. However hydrogen has two valence electrons.
In water H2O is stable substance which is formed when 2 hydrogen atom reacts with 1 oxygen atom. Solution for H3O Valence electrons. Now lets to find the hybridization of H3O using this method In hydronium ion the central atom is oxygen and it has 6 valence electronsThus by the method V 6.
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. Center atom of PO 4 3-ion. Lone Pairs around central atom 1.
Thus by the formula V 6. The chemical formula Acetic Acid CH3COOH. H3O NH4 L S032 CHCI.
Of valence electron in water is 8 and in oxygen it is 6. Lone Pairs Single or multiple bonds around the central atom 4. Sulfur has 6 valence electrons in its outer shell.
Three hydrogen atoms are bonded to oxygen so the choice of the monovalent atoms M 3As it is a cationic molecule thus C 1. Total number of valence electrons Valence electrons of Hydrogen Valence electrons of SulfurValence electrons of Hydrogen. So H ½ 6 3 1 4.
Total valence electrons pairs. So H ½ 6 3 1 4. Therefore we only have 8 valence electrons for the H3O Lewis structure.
H 3 O is also known as the Hydronium Ion and is very important in acid-base chemistry. Each of the hydrogen atoms contributes 1 valence electron while oxygen contributes 6. Oxygen has a total of 6 valence electrons and hydrogen contains 1 valence electron.
Hence we will multiply the number by 4. A step-by-step explanation of how to draw the H3O Lewis Structure. In oxygen Atomic number of Oxygen.
Oxygen has 6 valence electrons in its outer shell but as we know there are 4 oxygen atoms in this molecule. Oxygen share its 3 valence electrons with 3 hydrogen atoms and left with 3 valence electrons. For PO 4 3-ion Total pairs of electrons are 16.
Therefore it would has an octet in its valence shell. Remember that hydrogen H only needs two valence electrons to have a full outershell. Total electron pairs are determined by dividing the number total valence electrons by two.
Lewis Structure electron groups electron. Now lets find the hybridization of H3O using this formula In hydronium ion the central atom is oxygen and it has 6 valence electrons. Crude Sketch Calculations of valence electrons of bonds etc.
So now there are 24 valence electrons for all oxygen atoms. There are 8 valence electrons for the H3O Lewis structure. To be the center atom ability of having greater valance is important.
I also go over hybridization shape and bond angle. Since there are 3 H atoms and 1 atom the total number of valence electrons from the atoms alone is 9. Oxygen has 6 valance electrons.
I quickly take you through how to draw the Lewis Structure of hydronium ion H3O. Hydrogen atoms only need 2 valence electrons to have a full outer shell. It has 11 protons and 8 valence electrons.
Hence the valence for the acetic acid is 24. Total valence electrons 5 24 3 32. H3O is an important compound in.

Electron Dot Structure Of Hydronium Ion Brainly In

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
Electron Dot Structure Of Hydronium Ion

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
What Is The Shape Of H3o Ion Quora

Chemistry Chemical Bonding 22 Of 35 Lewis Structures For Ions Hydronium Ion H3o Youtube

Calculating Nh3 Formal Charges Calculating Formal Charges For Nh3 Ammonia Youtube

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

What Is The Shape Of H3o Ion Quora

9 26 06 Reading 8 6 Bond Length P Exceptions P Ppt Video Online Download

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

What Is The Molecular Geometry Shape Of The Bf4 Ion Quora
What Is The Shape Of H3o Ion Quora

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
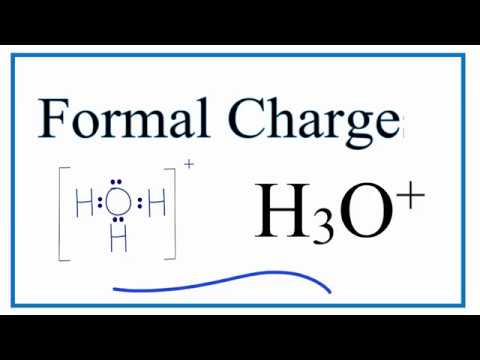
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube
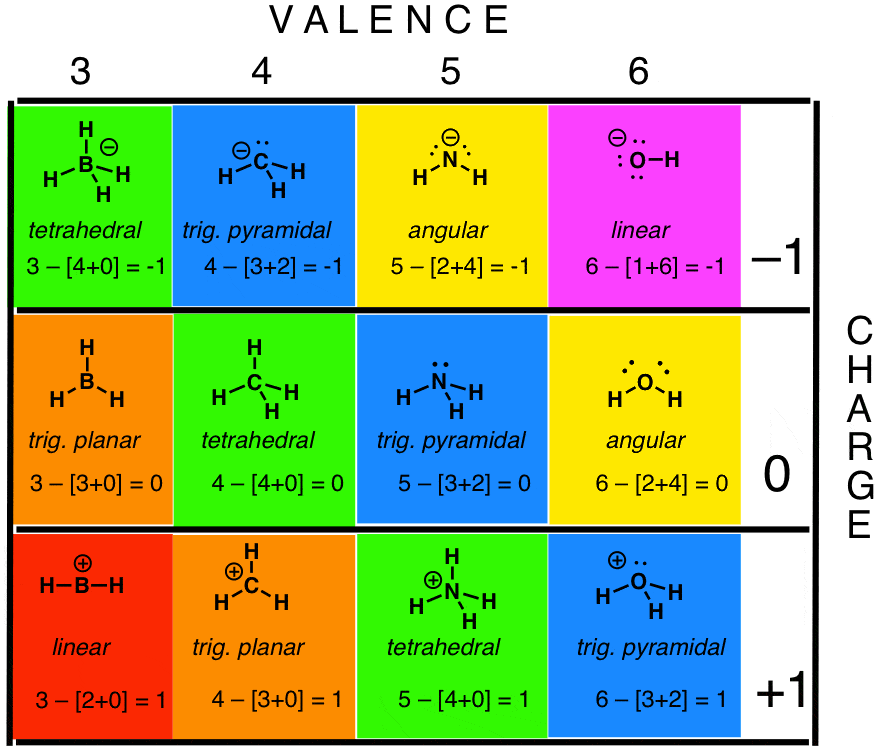
How To Calculate Formal Charge