Is Nh4+ Sp3 Hybridized
They have trigonal. How is NH4 formed.

Nh4 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Hybridization Number of Ion Pairs Number of Sigma Bonds.

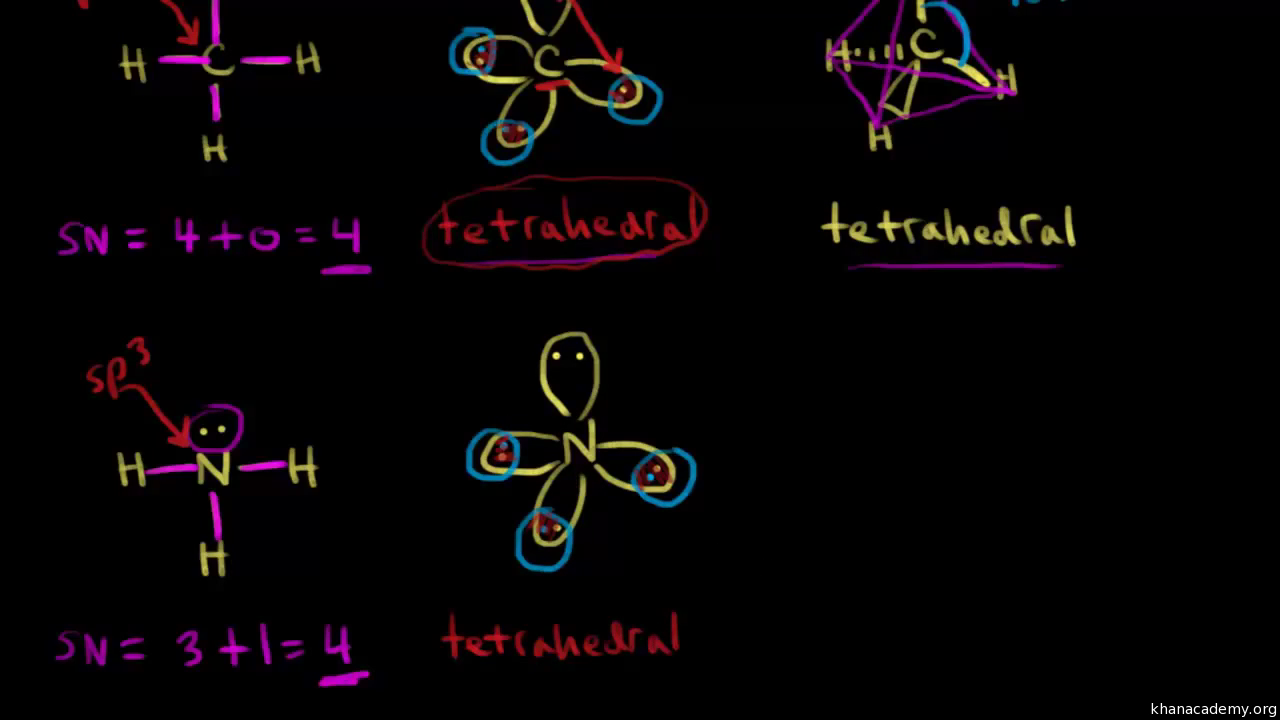
Is nh4+ sp3 hybridized. Since Ammonium has 0 ion pairs and 4 sigma bonds the hybridization value is 4. Therefore the configuration of NH4 is SP3. What is the hybridization of the central atom in NH4.
It allows the electron shape to become tetrahedral so the electrons can be farther apart. Of valence e- in the central atom b is no. Notice that the three hybrid view the full answer.
The hybridization of any atom in a molecule can be calculated by the summation of the number of sigma bond and lone pair of electrons. All electron containing atomic orbital of outer most shell of N atom are participate in hybridization. In N O 3 the central N atom has 3 bonding domains one single bond and two double bonds and zero lone pairs of electrons.
One 2s orbital and three 2p orbitals mix to form four sp3 hybrid orbitals. There is a best shortcut method to find hybridisation first count sorounding atom leaving main atom for example in NH4 N is main atom and there is 4 sorrounding atom then according to formula H equals SAhalfG-VE for -ve charge and -E for ve charge where H is hybridation SA is sorounding atom G is valence electron for main atom V is valency of all sorrounding atoms E is no of charge In NH4. The Hybridization of N atom in N O 3 N O 2 and N H 4 is s p 2 s p s p 3 respectively.
In the ammonium ion NH4 what percentage of p character do the hybrid orbitals of nitrogen possess. The steric number can be found by adding the number of bonded atoms and then num. Its hybridization state is therefore sp3.
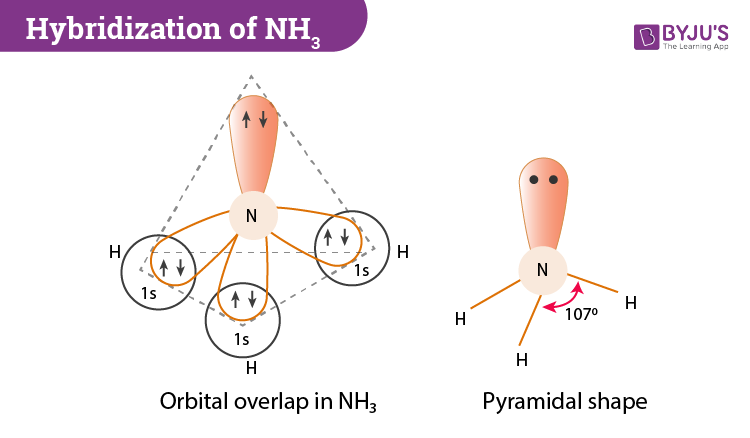
The hybridization is sp. Ethane C 2 H 6 methane. Further if we look at the NH 3 molecule you will notice that the three half-filled sp3 orbitals of nitrogen form bonds to hydrogens three atoms.
The nitrogen atom forms 4 bonds and has 0 lone pairs so it needs 4 hybrid orbitals. Each sp 3 hybrid orbital has 25 s character and 75 p character. The carbon atom in ethane is sp3 hybridized.
Sp 3 d Hybridization. As a result hybridization of central N atom is sp3. Therefore the hybridization of NH4 is sp3.
In chemistry orbital hybridisation or hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theoryFor example in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence. The C atom can make one more bond to complete its octet. The geometry is linear.
Using the formula we get 2 1 5 4 1 4. The carbon atom in ethane is sp2 hybridized. One 2s orbital and two 2p orbitals mix to form three sp2 hybrid orbitals.
Example of sp 3 hybridization. This _____ atom has the following hybridized orbitals. To find the hybridization for NH4 well first determine the steric number.
In hybridization carbons 2s and three 2p orbitals combine into four identical orbitals now called sp 3 hybrids. Where N is no. Who are the experts.
The bonds between carbon and hydrogen can form the backbone of very complicated and extensive chain hydrocarbon molecules. Which of the following has an atom that is sp2 hybridized. Hybridization of NH4 CHEMICAL BONDING hybridization and shapes of nh4nh4studybibhaschemical bonding class 11chemical bonding video lecturehybridisa.
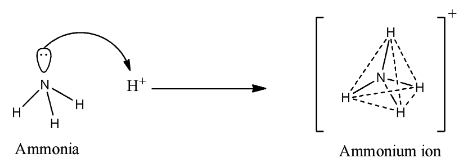
The tetrahedral set of sp3 is obtained by combining the 2s and three 2p orbitals. Now ammonia forms ammonium cation by the donation of lone pair on nitrogen atom. The C atom can male on more bond to complete its octet.
Hybridisation is sp3 of NH4 because in outermost shel it have 5 valence electrons and big positive charge and it have four hydrogen atom that is attached to it so formula to calculate hybridisation is 1254-1 is equal to 4 so it is sp3. The sp3 orbitals have 25 s character and 75 p character. It seems that atoms with 4 or more valence electrons usually have sp3 hybridization.
So in ammonium cation the N -atom formed 4 sigma bond with the four H-atom. Of atoms surrounding it c is the total charge on the atom. The central atom in the NH4 ion is ____.
What is the hybridization of NH4. In N O 2 the central N atom has 2 bonding domains one single bond and one double bond and zero lone pairs of. Experts are tested by Chegg as specialists in their subject area.
There have four electron containing orbital in outer most shell of N atom. The simplest of these is ethane C 2 H 6 in which an sp 3 orbital on each of the two carbon atoms joins. Here we can use the formula 2 1 N B c to find the hybridization.
Another way of identifying the hybridization of an atom is by the following formula. Sp 3 d hybridization involves the mixing of 3p orbitals and 1d orbital to form 5 sp3d hybridized orbitals of equal energy. The C atom has two unhybridized p atomic orbitals.
The nitrogen atom in NH3 is sp3 hybridizedIf we consider the Lewis structure of ammonia the four electron pairs around the nitrogen atom require a tetrahedral arrangement. It also accounts for the bond angles in NH3 of 107 slightly different than normal tetrahedral because of the lone pair of. There are 2 sigma and two pi bonds.
In NH4 ion there is no pi bond. During the formation of ammonia one 2s orbital and three 2p orbitals of nitrogen combine to form four hybrid orbitals having equivalent energy which is then considered as an sp 3 type of hybridization. However the fourth sp3 orbital that is present is a nonbonding pair of hybridized.
CH3 -CH3 NH3 Please explain why. Expert Answer 100 19 ratings The notation sp2 indicates that the hybrids are mixtures of onesorbital and twoporbitals. The angle between the sp3 hybrid orbitals is 10928 0.
What is the shape of NH4.

Hybridization For Nh4 Description Of Hybrid Orbitals For Nitrogen Sri Lanka Vlip Lv

Lewis Structure Hybridization Nh4 Youtube
What Is Hybridization Of Nh3 Quora

The 3 D Structure Of Ammonium Ion Nh4 Youtube
Steric Number Video Bond Hybridization Khan Academy

Hybridization Of Nh4 Chemical Bonding Hybridization And Shapes Of Nh4 Youtube

Why The Formation Of Ph4 Is Difficult Compared To Nh4 Chemistry Stack Exchange
N Atom In Nh4 Ion Involves The Hybridization A Sp Class 11 Chemistry Jee Main
Hybridization Of Nh3 Ammonia Hybridization Of Nitrogen In Nh3
What Is The Hybridisation Of Nh4 Quora
What Is The Hybridization Of The Central Atom In The Chegg Com

Sp Hybridization Hybrid Orbitals Chemical Bonds Video Khan Academy
What Is The Hybridisation Of Nh4 Quora
What Is The Hybridisation Of Nh4 Quora

Why Is The Bond Angle The Nh4 Greater Than Nh3 Quora
What Is The Hybridisation Of Nh4 Quora

Hybridization For Nh4 Description Of Hybrid Orbitals For Nitrogen Youtube
Hybridization Easy Chemistry Nurlisa Hidayati S Page Facebook

Chemical Bonding Molecular Structure Ppt Video Online Download