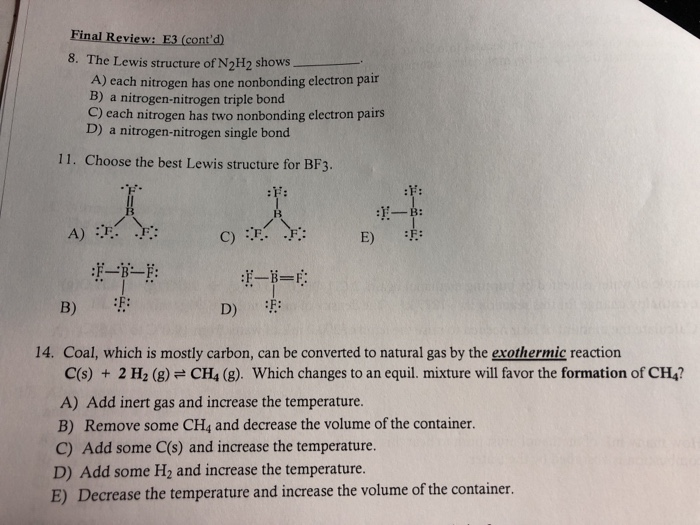
Lewis Structure Of N2h2 Shows
Ion is A 0. A nitrogen-nitrogen single bond.

The Lewis Structure Of N2h2 Shows A Each Nitrogen Chegg Com
Each nitrogen has one nonbinding electron pair Lewis structures are diagrams that show the bonds between the atoms of a molecule and the lone pair of electrons that might exist in a molecule.

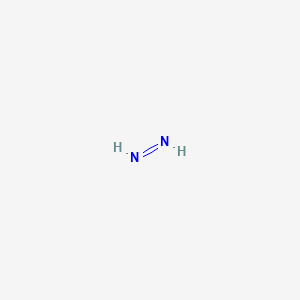
Lewis structure of n2h2 shows. The Lewis structure of N 2 H 2 shows ________. This is the N2H2 Lewis Structure. Each nitrogen has one nonbonding electron pair Question 2 Determine the mass in grams of potassium chloride 745 gmol that must be added to 45 g of water to make a 245 m solution.
The Lewis structure of N2H2 shows single bonds between the nitrogen and hydrogen atoms in the compound while the N atoms are connected via a double. A a nitrogen-nitrogen triple bond B a nitrogen-nitrogen single bond C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs. We have two Hydrogens.
A nitrogen-nitrogen triple bond. A a nitrogen-nitrogen triple bond B a nitrogen-nitrogen single bond C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs E each hydrogen has one nonbonding electron pair. The Lewis structure of N2H2 shows _____ asked Dec 30 2019 in Chemistry by Pirlo.
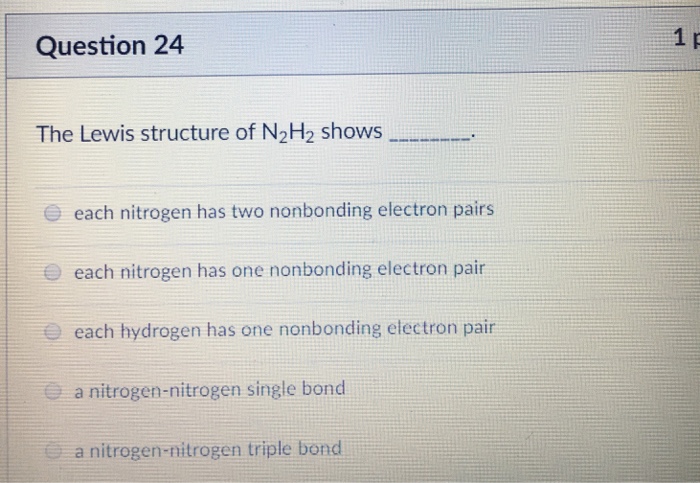
B 6 The Lewis structure of the C032. The Lewis structure of N2H2 shows _____. Each nitrogen has one nonbonding electron pair.
Enter your answer to 2decimal places. Further Explanation Lewis structures can be drawn for each covalently bonded molecule as well as coordination compounds. 11 The Lewis structure of HCN H bonded to C shows that _____ has _____ nonbonding electron pairs.
Three single bonds and four unshared pairs 3. The Lewis structure of N2H2 shows A each nitrogen has one nonbinding electron pair B each nitrogen has two nonbinding electron pairs C a nitrogen-nitrogen single bond D a nitrogen-nitrogen triple bond E each hydrogen has one nonbonding electron pair 2 In the nitrite ion NO2- A both bonds are the same B both bonds are single bonds C there are 20 valence. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist.
A0 B1 C2 D3 EThis cannot be determined from the data given. Relevant worked examples were given in the following articles. Chemistry I For Dummies-John T.
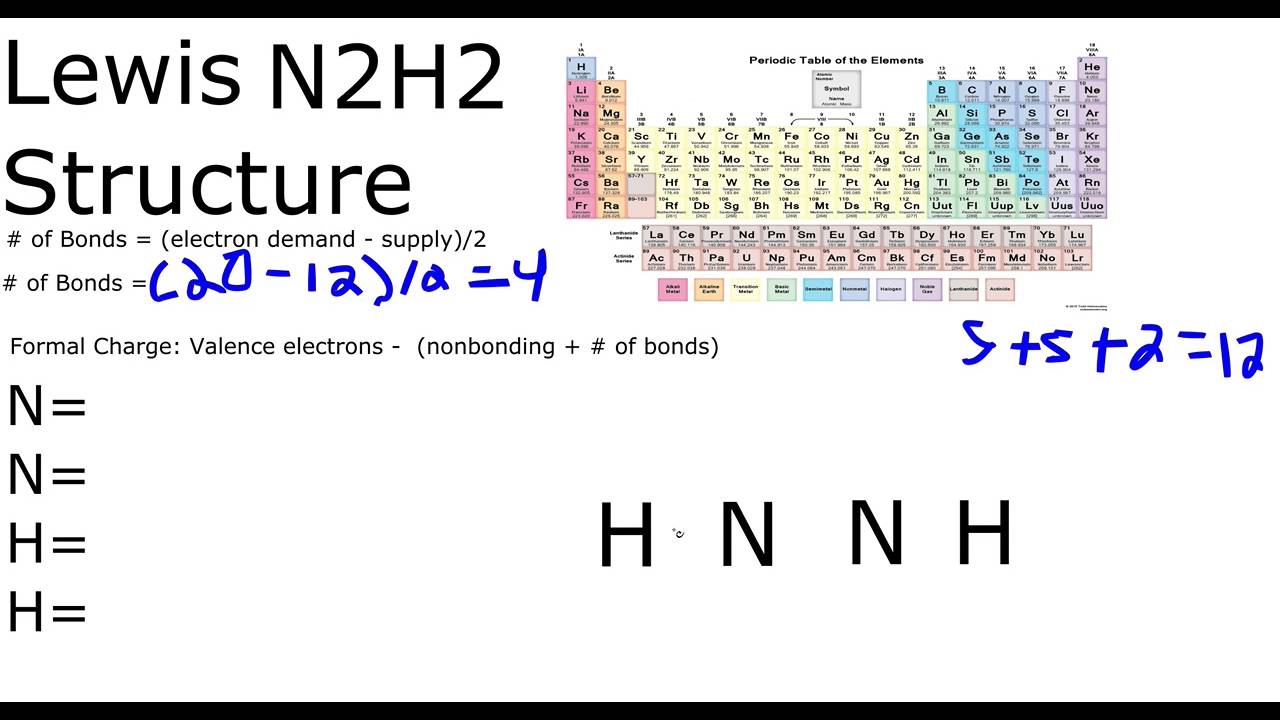
AC 1 BN 2 CC 2 DN 1 EH 1 12 The Lewis structure of N2H2 shows _____. A a nitrogen-nitrogen triple bond B a nitrogen-nitrogen single bond C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs E each hydrogen has one nonbonding electron pair. Nitrogen is in Group 5 or fifteen on the periodic table so it has five valence electrons but we have two of them.
Moore 2015-08-10 Now you can score higher in chemistry Every high school requires a course in chemistry for graduation and many universities require the course for majors in medicine engineering biology and various other sciences. The Lewis structure of N2H2 shows ________. 10 The Lewis structure of AsH3 shows _____ nonbonding electron pairs on As.
Hydrogen is in group 1 it has one valence electron. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. This is the n2h2 lewis structure.
The Lewis structure of N 2 H 2 shows _____. The Lewis structure of N2H2 shows A a nitrogen nitrogen triple bond B a from CHEM 1411 at Dallas County Community College. A step-by-step explanation of how to draw the N2H2 Lewis Dot Structure Dinitrogen dihydrideFor the N2H2 structure use the periodic table to find the total.
Transcribed image text. The Lewis structure of N2H2 shows A each hydrogen has one nonbonding electron pair B each nitrogen has one nonbonding electron pair C a nitrogen-nitrogen triple bond D each nitrogen has two nonbonding electron pairs E a nitrogen-nitrogen single bond. However its quite common to find it in organic compounds.
Group of answer choices a nitrogen-nitrogen single bond each hydrogen has one nonbonding electron pair each nitrogen has one nonbonding electron pair each nitrogen has two nonbonding electron pairs a nitrogen-nitrogen triple bond. Download How To Draw Lewis Dot Structure For N2h2 U Can. So ten plus two gives us a total of twelve valence electrons.
11 7 The Lewis structure of N2H2 shows A a nitrogen-nitrogen triple bond B each hydrogen has one nonbonding electron pair C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs E a nitrogen-nitrogen single bond 8 In the most stable resonance. The lewis structure of n2h2 shows c. The Lewis structure of N2H2 shows _____.
Question 24 The Lewis Structure Of N2h2 Shows Each Chegg Com

The Molecule Called Diazene Has The Formul Clutch Prep

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
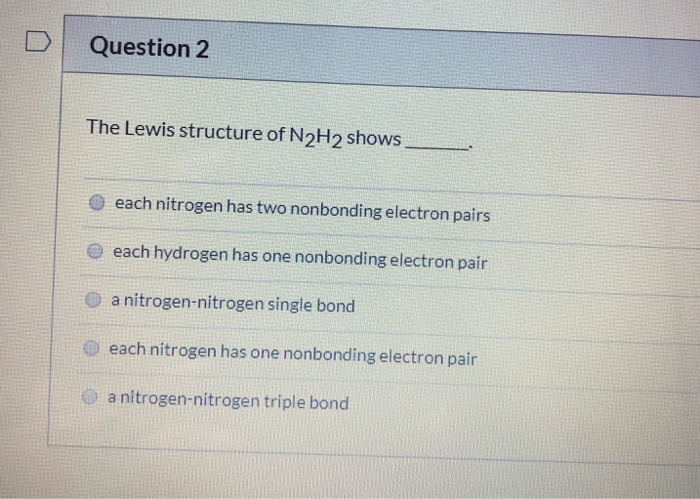
Question 2 The Lewis Structure Of N2h2 Shows Each Chegg Com
Final 8 The Lewis Structure Of N2h2 Shows A Each Chegg Com

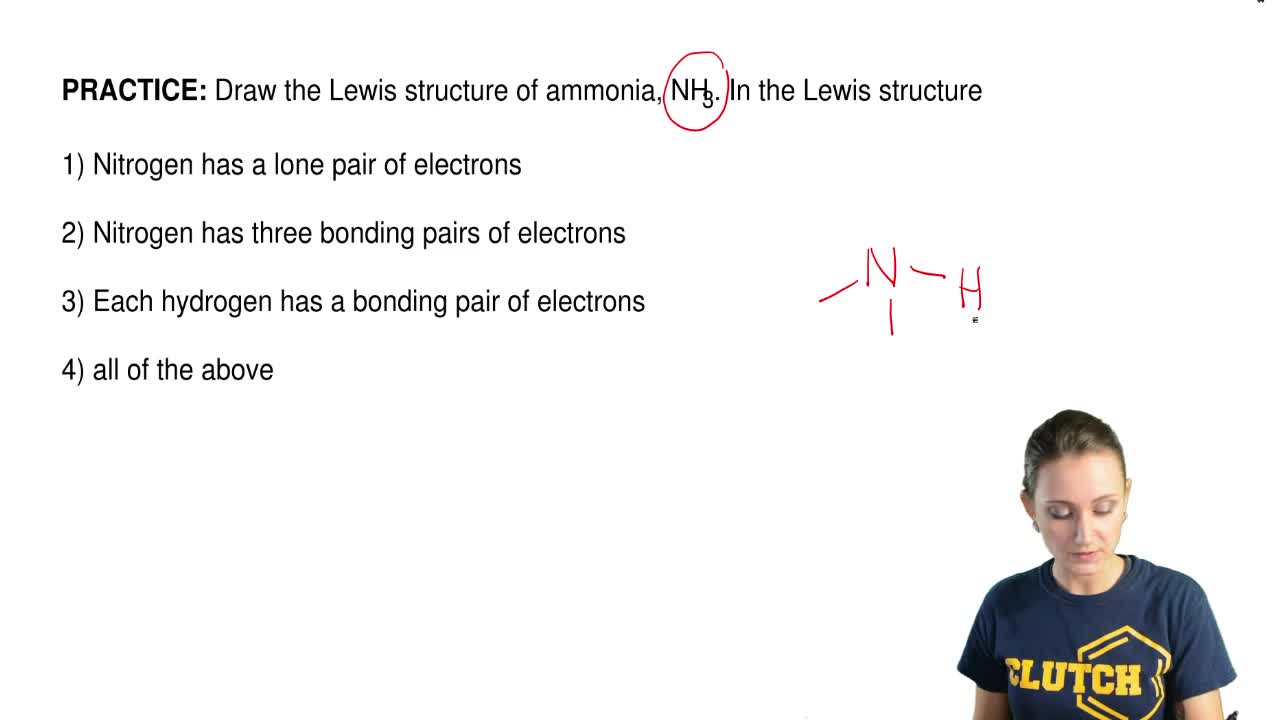
Draw The Lewis Structures Of N2h4 N2h2 And N2 Draw The Molecules By Placing Atoms On The Grid And Brainly Com
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

Welcome To Quilava S Blog Chemistry Education Structural Formula Nurse Drawing
Solution Draw The Lewis Structure Of Ammo Clutch Prep

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

The Lewis Structure Of N2h2 Shows A A Nitrogen Nitrogen Triple Bond B A Nitrogen Nitrogen Brainly Com

In The Lewis Structures Of N2h2a There Is Clutch Prep

Solution The Lewis Structure For N2h2 Hh Clutch Prep
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
Question 1 The Lewis Structure Of N2h2 Shows Each Chegg Com

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

Chem Catalyst These Diagrams Are Called Lewis Dot