Possible Lewis Structure For Cocl2
Since there is only one possible lewis structure C2H2 does not have resonance. C4O6Cl2x714 Total24 Put carbon in the center.

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas
Since the double bond can be placed in more than one place without rearranging the atoms COCl2 exhibits resonance.

Possible lewis structure for cocl2. 2In this compound carbon is the central atom. Construct the Lewis structure model for the covalent compound phosgene COCl2 using the following steps. Does COCl2 have resonance structures.
Solution Set 1 Msu Billings Pages 1 6 Text Version. 3 A Draw the Lewis structure for phosgene COCl2. The Lewis structure of the CoCl2 molecule shows that there is a double bond between the O and the central C atom.
Therefore the dot resonance structures of COCl 2 Lewis structures of COCl2 are as follows. If any resonance structures are possible Lewis structures then. 3Add electron pairs to show the bonds between the atoms.
The first with a double bond between carbon and oxygen the second with a double bond between carbon and one chlorine and the third with a double bond between carbon and the other chlorine. A tetrahedral D either tetrahedral or. A molecule has resonance if more than one lewis structure can be drawn for that molecule.
D Sketch the molecule beginning with the central. Four electrons it represents a double bond. Calculate the total valence electrons in COCl 2 molecule.
Moreover there exist many lone pairs which do not alter the molecular geometry but make the molecule polar. Lewis dot structure 2 is the more probable most stable since there is no charge separation. Check me out.
Show the formal charges for all elements in each structure and indicate how you arrived at them. For the molecule COCl 2 write the possible Lewis Dot structures and indicate the correct one based on formal charge arguments. Youll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom.
Arrange the atomic cores appropriately in the space below. Alternatively a dot method can be used to draw the lewis structure of COCl 2. B Using VSEPR Theory what is the electron geometry and molecular geometry of the compound.
Based on the best Lewis structure for COCl2 what is the formal charge on carbon. Ib Diploma Chemistry Hl Textbook Pdf Pdf Document. The most stable structure for COCI 2 is Structure A.
Put the H here--always goes on the outside--and then the Oxygen and the Chlorine. A double bond represents a slightly larger electron domain which disrupts the ideal 120 CI-C-Cl bond angle making it smaller. Therefore this is the correct Lewis Structure representation of COCl2.
List all possible molecular geometries shapes for a nonpolar molecule with the formula AX4. Carbonyl fluoride COF2 is a toxic and inflammable compound whose Lewis structure determines the presence of a double bond between the carbon and oxygen atoms and single bonds between the carbon and fluorine atoms. Please label both geometries.
In this section we will learn about another concept of chemistry. Which Is An Acceptable Lewis Structure Of Clutch Prep. Solved 8 45 Draw Lewis Structures For The Following Molec.
See the Big List of Lewis Structures. Sharing of a single electron pair represents a single bond whereas when two atoms share two electron pairs ie. In which one of the following species is the central atom the first atom in the formula.
O 4 electrons from lone pairs plus 2 electrons from bonds 6 electrons. In the Lewis structure for COCl 2 there are a total of 24 valence electrons. C Using Valence Bond theory what is the hybridization of the carbon atom.
Answered A Solution Of 0 079 M Cobr2 Is Bartleby. Which is the most stable Lewis structure for COCl2. How many sigma and pi bonds are present.
For the HOCl Lewis structure there are a total of 14 valence electrons. This is the HOCl Lewis structure. Does C2H2 have resonance.
C 0 lone pairs plus 4 electrons from bonds 4 electrons. Lewis dot structure of CO Cl 2. Chemical Bonding Lewis structure Lewis symbol.
Calculating formal charge will show that the carbon-oxygen double bond structure. Since the double bond can be placed in more than one place without rearranging the atoms COCl2 exhibits resonance. In the COCl 2 Lewis structure Carbon is less electron electronegative than Oxygen and goes in the center of the Lewis structure note that Hydrogen atoms always go on the outside.
For HOCl we have 1 valence electron plus 6 for the Oxygen and Chlorine has 7 for a total of 14 valence electrons. 1 The total number of valence electrons in COCl2 is ____. Its Lewis structure can be drawn 3 ways.
Cl 6 electrons from lone pairs plus 1 electron from a bond with C 7 electrons. A 0 B 1 C -1 D 2 E -2. Write these charges next to the atoms in the Lewis structure.

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar
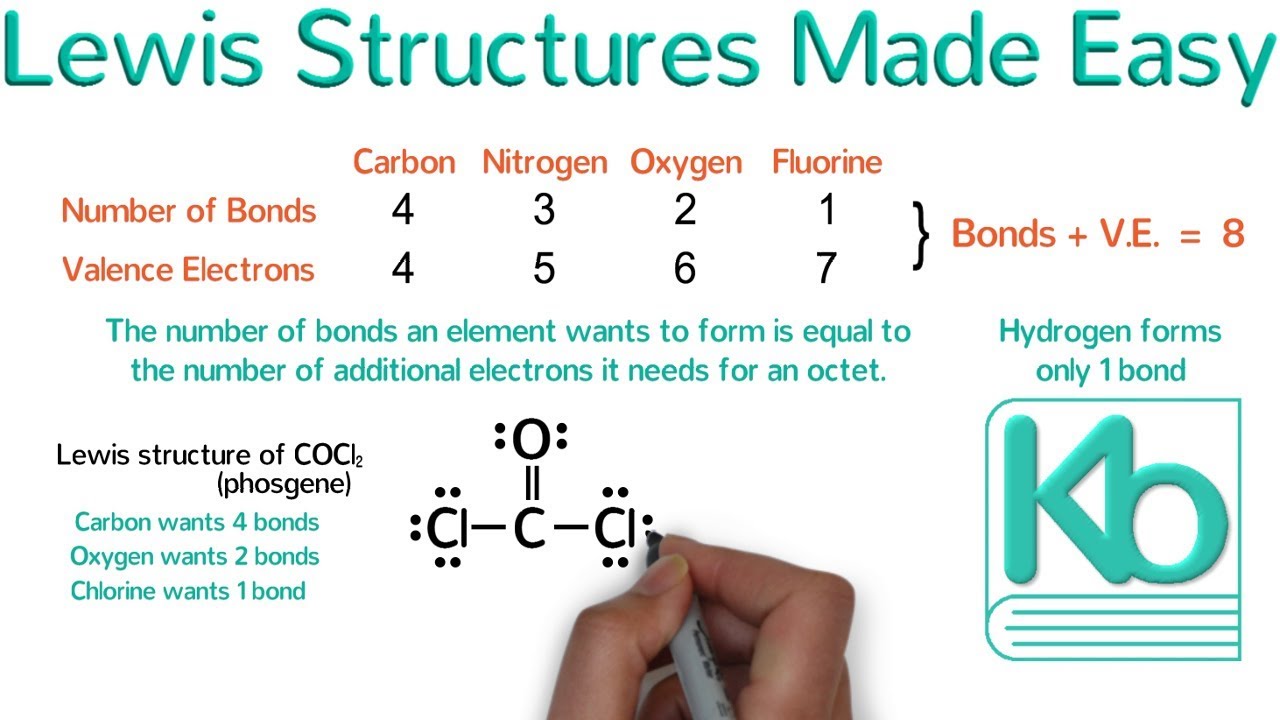
Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar