So3 Lewis Structure Explanation
Lewis structure is a graphical representation of molecules that shows the electrons and bonds that exist in a particular compound. Lewis Structure of SO3 Valence.

How To Determine The Lewis Dot Structure Of So3 Quora
C I3 D SO3.

So3 lewis structure explanation. Sulfite ion sulphite ion SO 3 2-Sulfite ion is one of the oxyanion of sulfur. It is prepared on an industrial scale as a precursor to sulfuric acid. SO 3 is named Sulfur Trioxide.
The oxygen atom of the water contains two lone pairs so water is a Lewis base and the sulfur has three electrons which make SO3 Lewis acidic. Sulfur trioxide alternative spelling sulphur trioxide also known as nisso sulfan is the chemical compound with the formula SO 3It has been described as unquestionably the most important economically sulfur oxide. Lets do the SO3 2- Lewis structure.
In that case ceSO_3 contains one double bond and two single bonds which is why people tend to list the overall bond-order as 133. There are 32 valence electrons available for the Lewis structure for SO 3. For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up herethere are two extra valence electrons.
How come lewis structure for SO3 has 6 double bonds on the S atom. For the most stable Lewis. 1 We are given SO3 S O 3 molecule.
In each of the three structures in the middle S has a formal charge of 1 and one of the O atoms has a formal charge of -1. SO3 has 24 valence electrons. As a simplistic explanation the above sources state that the lewis structure of ceSO_3 contains a 2 charge on the central sulfur and negative charges on two of the three bonded oxygen atoms.
This representation has been very useful in fields of chemistry as organic and synthetic chemistry. In the bottom structure all atoms have a formal charge of zero. In each of them S has a formal charge of 2 and two of the O atoms have formal charges of -1.
When you draw the Lewis structure you first get the three structures at the top. Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. Calculate the formal charge for each atom in molecules A and B.
Lewis structure of sulfite ion is drawn in this tutorial step by step. Follow the below instructions. Answer the following questions.
You can easily determine that its a base or acid. Sulfur trioxide exists in several forms - gaseous monomer crystalline trimer and solid polymer. Two possible Lewis structures for the sulfur trioxide molecule SO3 molecule are shown in the figure below.
One appliance for this Lewis structures is the explanation of unreactivity in some compounds with resonance structures. E SO3 2 I dont understand what this question is really talking about. 6 3 x 6 24.
In which one of the following is the best Lewis structure a resonance structure. The answer is E. It is a form of pollution.
SO3 is a trigonal planar molecule that is non-flammable. Sulfur trioxide has two elements oxygen and sulfur. SO 3 is the primary contributer to acid rain in the atomsphere.
Here sulfur in the center because of its lowest electron capability and three oxygen around it. Before and after the reaction count the hydrogens on each substance. But I dont understand.
One bond represents 2 electrons. How come lewis structure for SO3 has 6 double bonds on the S atom. S 0 -S2 -O.
Determine the most stable Lewis structure and explain why. Wondering what the explanation is behind this and how I know when to draw more then 8 electrons for an atom. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
A SCO C central atom B BF3. That gives more then 8 electrons on the atom. Nov 25 2018 - A step-by-step explanation of how to draw the SO3 Lewis Dot Structure Sulfur trioxideFor the SO3 structure use the periodic table to find the total number.
Sulphur has 6 electrons and eac oxygen atom provides 3. Sulfur brings 6 and oxygen brings 3 each. How can something be a resonance structure.
That gives more then 8 electrons on the atom. Sulphur is the central atom of sulphate that is bind to other three oxygen atoms. Find an answer to your question Draw the Lewis dot structure for SO3 arya28singh02 arya28singh02 06092019 Chemistry Secondary School answered Draw the Lewis dot structure for SO3 2 See answers ARCE ARCE.
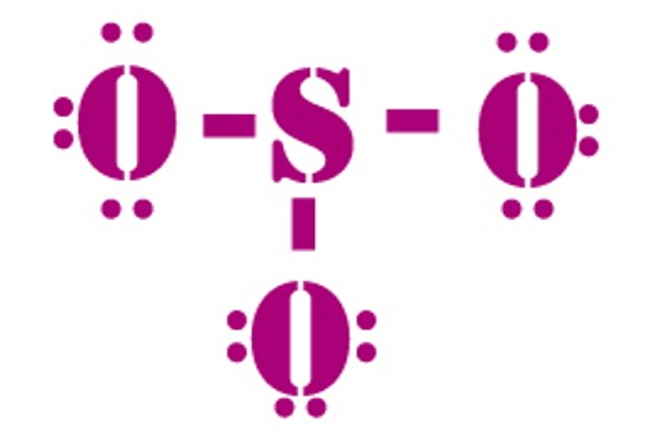
So3 Molecular Geometry Lewis Structure And Polarity Explained

So3 Molecular Geometry Lewis Structure And Polarity Explained

So3 Lewis Structure Sulfur Trioxide Youtube

Lewis Structure Of So3 Sulfur Trioxide Youtube

How To Determine The Lewis Dot Structure Of So3 Quora

H2s Lewis Structure Chemistry Worksheets Electron Configuration Teaching Chemistry

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 Molecular Geometry Writing Systems Biology

So32 Molecular Geometry Shape And Bond Angles Molecular Geometry Chemistry Help Molecular

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
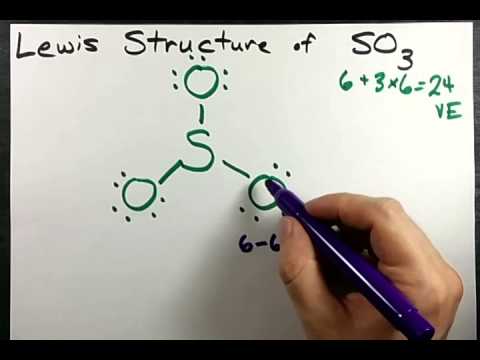
Lewis Structure Of So3 Sulfur Trioxide Youtube

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube
So3 Molecular Geometry Lewis Structure And Polarity Explained

How To Determine The Lewis Dot Structure Of So3 Quora

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube

126 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube Chemistry Classroom Science Chemistry Chemistry
