Draw The Lewis Structure Of Brf3
How many lone pairs are on the central atom of BrF3. Find step-by-step Chemistry solutions and your answer to the following textbook question.

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
There are a total of 28 valence electrons for the BrF 3 Lewis structure.
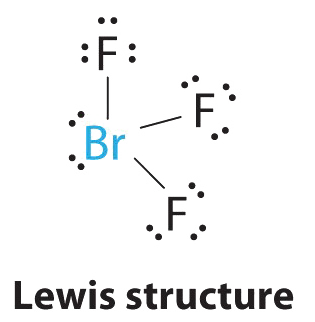
Draw the lewis structure of brf3. A three-step approach for drawing the BF3 Lewis structure can be used. As three electrons out of seven form a bond with the valence electrons in the Fluorine atom there are four nonbonding electrons on the central atom of BrF3. Thats six valence 2 4 6.
Drawing the Lewis Structure for BrF 3. Bromine trifluoride chemical formula is BrF3. Draw the Lewis structure for the BrF3 molecule.
Sketch the Lewis structure of the molecule BrF3 showing in detail. A the electron pairs on Br b molecular geometry c formal charge on Br d the polarity of the molecule polar or non-polar 2. We have a total of 28 valence electrons for the BrF3 Lewis structure.
By signing up youll get thousands of step-by-step. As per the common rule we keep the least electronegative element in the center. The carbon iodine and hydrogen elements come as the member of the carbon halogen and hydrogen family groups from the periodic table respectively.
Draw a Lewis structure for each of the following molecules or ionsa BrF3b c XeO2F2d. Well put single bonds between the Fluorines and the Bromine. A BrF3 b ICl2- c BeF2.
In the BrF 3 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure. Br and F are both halogens belonging to group 7 in the periodic table. Draw the Lewis structure for BrF3 in the window below.
Solutions for Chapter 8 Problem 11PC. B Predict their electron-domain and molecular geometries. Both need 1 extra electron to complete their octets.
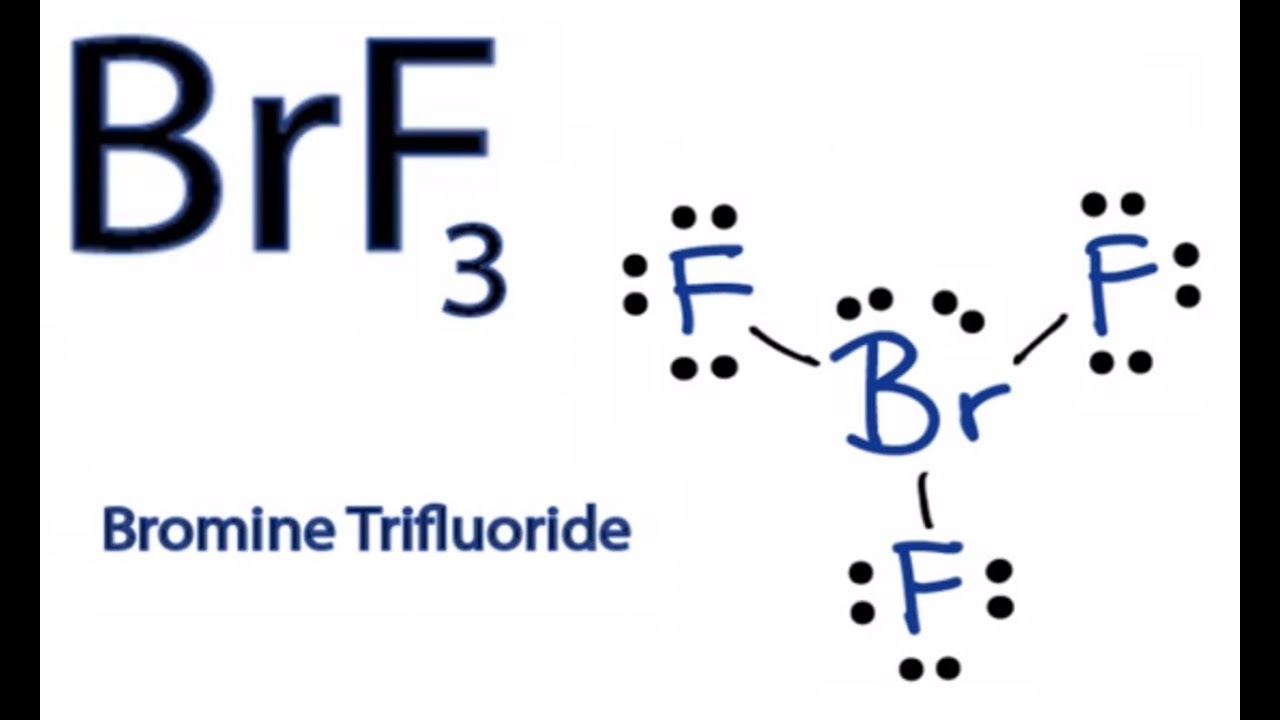
April 11th 2019 - This is the BrF3 Lewis structure We have a total of 28 valence electrons for the BrF3 Lewis structure Bromine is the least electronegative we ll put that at the center and then we ll put the Fluorines around the outside We ll put single bonds between the Fluorines and the Bromine That s six valence 2 4 6 valence electrons we ve used. Draw the Lewis structure of BrF3 and include the lone pairs. Bromine is the least electronegative well put that at the center.
Draw The Lewis Structure For BrF3 In The Window Below And Then Answer The Questions That Follow. After determining how. By signing up youll get thousands of step-by-step solutions to your.
Here in this post we described step by step method to construct CH3I Lewis Structure. C Assign oxidation numbers and formal charges to each atom. Is BrF3 Polar Or Nonpolar.
Here in this post we described step. In the Lewis structure for BrF 3 there are a total of 28 valence electrons. Chemistry QA Library i ASF3 ii BrF3 iii ClO3 iv BrO2 a Draw the Lewis structure for each of the following molecules or ions.
84 382 ratings play-rounded-fill. The Lewis structure of BrF3 will have three bonds between Br-F represented by lines and four nonbonding electrons represented as four dots on the Bromine atom. Include all lone pairs of electrons.
Give the number of electrons in each species. Drawing CH3I Lewis Structure is very easy to by using the following method. These species do not obey the octet rule.
Get solutions Get solutions. A step-by-step explanation of how to draw the BF3 Lewis Dot Structure Boron TrifluorideFor the BF3 Lewis structure calculate the total number of valence. Therefore both of these elements will.
Draw a Lewis structure for each and state the type of octet-rule exception. The second step is to add valence electrons to the three fluorine atoms and the final step is to combine the step1 and step2 to get the BF3 Lewis Structure. Drawing BrF3 Lewis Structure is very easy to by using the following method.
Draw the Lewis structure for BrF3. This problem has been solved. Draw the Lewis structure of BrF3 and determine the bond angle between an equatorial F atom and an axial F atom 90º 90º 120º 120 1095º.
Steps to form BrF3 Lewis Structure Step 1. The first step is to sketch the Lewis structure of the BF3 molecule to add valence electrons around the boron atom. Draw the molecule by placing atoms on the grid and connecting them with bonds.
6 rows There are a total of 28 valence electrons for the BrF 3 Lewis structure. For the BrF 3 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. And then well put the Fluorines around the outside.

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep

Brf3 Electron Geometry Shefalitayal

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

What Is The Lewis Structure For Seobr 2 Clutch Prep
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
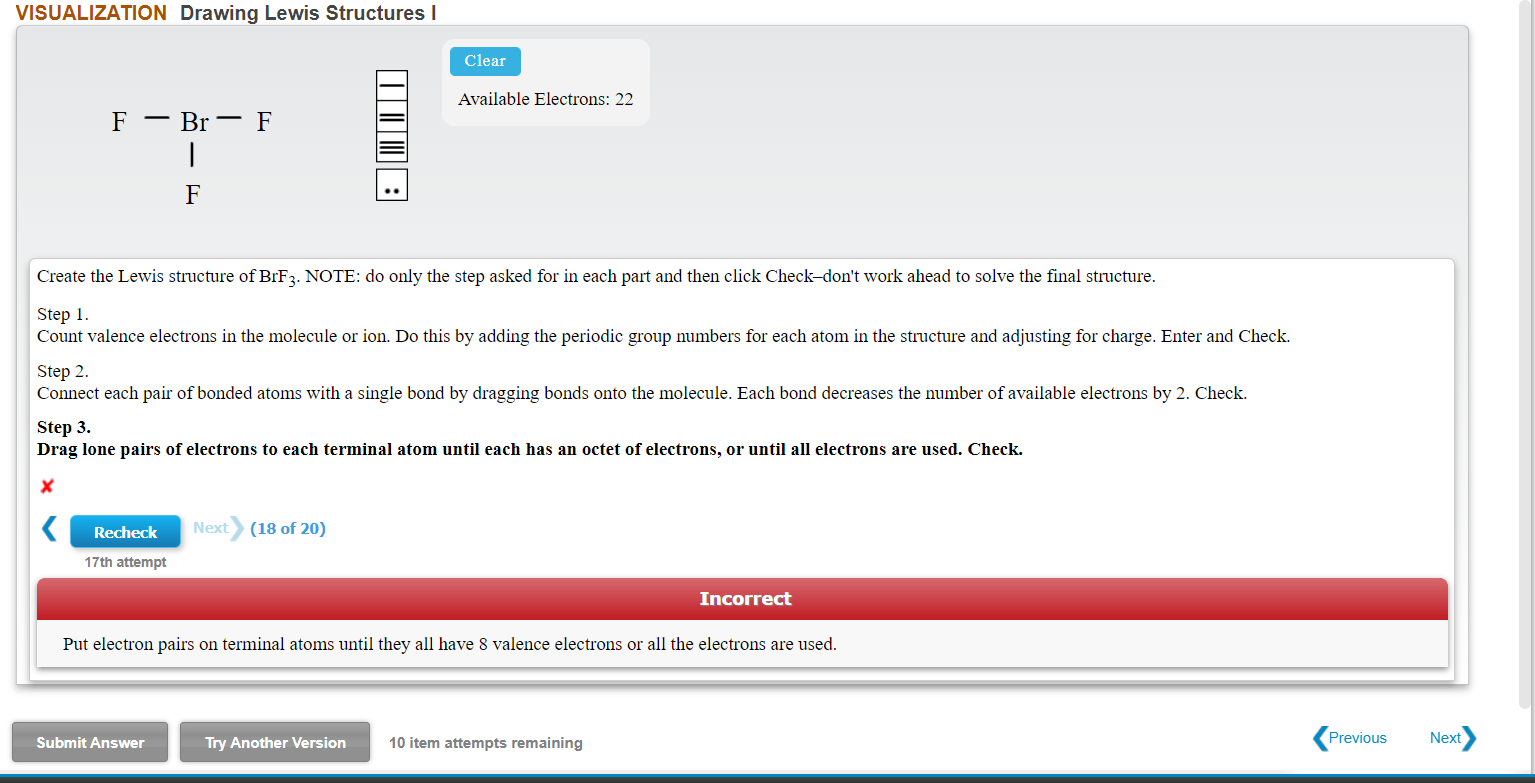
Create The Lewis Structure Of Brf3 Note Do Only The Chegg Com

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Predicting Geometry With Vsepr Chemistry Libretexts
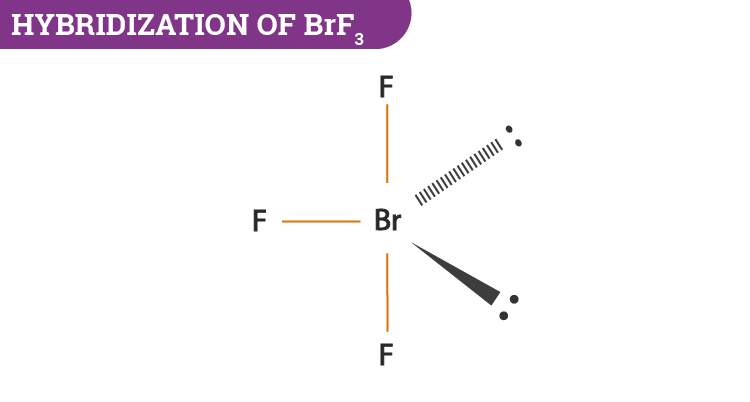
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride
What Is The Hybridization Of Brf3 Quora
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Bromine Trifluoride Youtube

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Solution Draw The Lewis Structure Of Brf Chemistry

Write Lewis Structures For Brf3 Clf5 And If7 Identify Those In Which The Octet Rule Is Not Obeyed Study Com
Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube
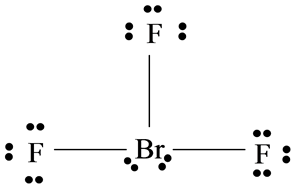
Solved Chapter 22 Problem 21e Solution Selected Solutions Manual General Chemistry 10th Edition Chegg Com