Sif4 Lewis Structure Molecular Geometry
The melting and boiling point of silicon tetrafluoride is -950 C and -903 C and hence it exists as a gas at room temperature. The Valence Shell Electron Pair Repulsion theory or VSEPR theory is one useful theory for predicting the geometries of molecules.

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
What is the Electron Pair Geometry.

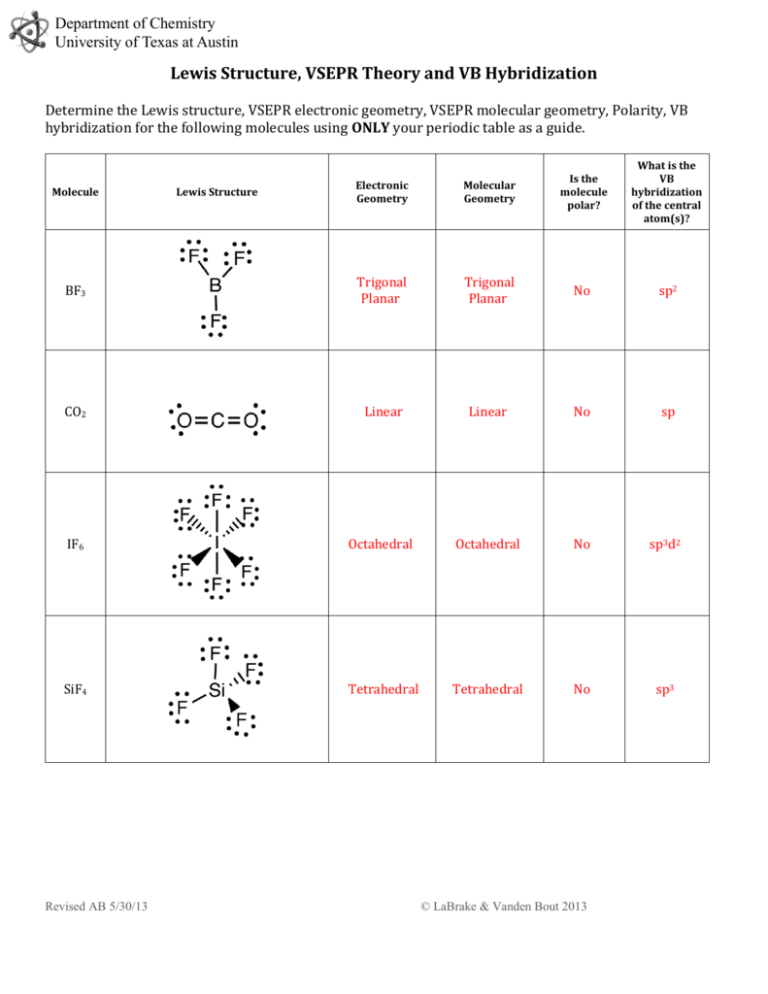
Sif4 lewis structure molecular geometry. In SiF4 the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. Sif4 lewis structure Within the context of VSEPR theoryyou can count electrons to determine the electron geometry parent geometry. Draw the lewis structure for COFH.
There are three lone pairs on each fluorine atom. CF4 is the molecular formula of Carbon Tetrafluoride and is the simplest fluorocarbon of all. SiF4 is not polar as the fluorines negative dipoles cancel each other out as the are all pulling away form the centre equally the centre being silicon which has a lower electronegativity than fluorine.
What is the Molecular Geometry. ClO3- Molecular GeometryShape Bond Angles Chlorate Ion Chlorate ion or ClO3- ion comprises one Chlorine atom that is in the center forming bonds with three Oxygen atoms. What types of bonds does the molecule have.
It forms a see-saw shape and has a trigonal bipyramidal molecular geometry. Moreover the compound is also called tetrafluoromethane as it. Vapor is heavier than air.
What is the Molecular Geometry. You can put these on the central Se atom. The electron arrangement and molecular shape are both linear.
What is the Electron Pair Geometry. Very toxic by inhalation. Electron And Molecular Geometry Revision 1 Admirable.
The molecular geometry of carbon tetra bromide is tetrahedral. P03- Valence electrons 5 Si02 Valence electrons 16. Hence Si atom is sp3 hybridized in this compound and SiF4 gets tetrahedral molecular geometry.
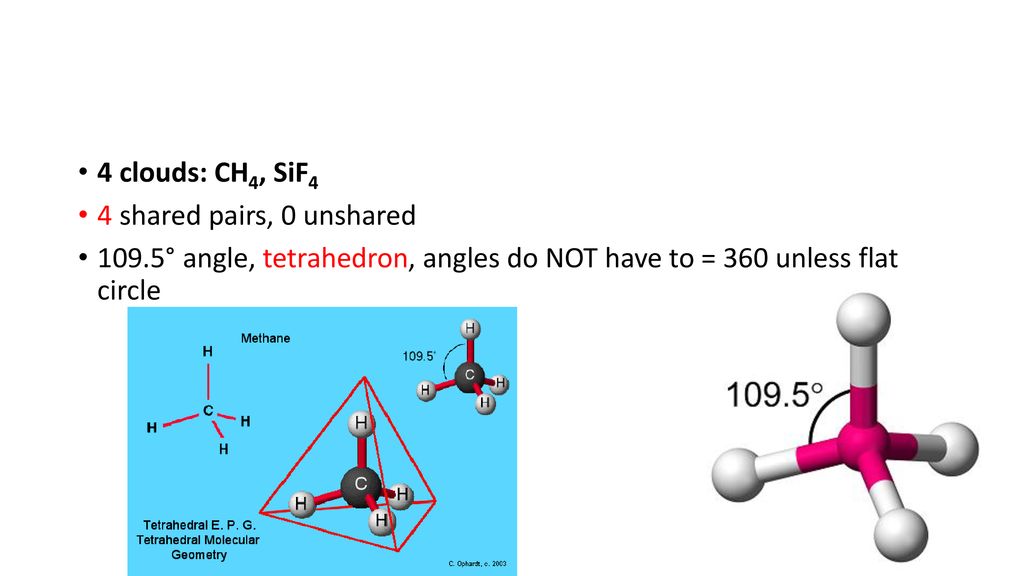
Experiment 12 Lewis Dot Structures and Molecular Geometry 12-2 Procedure for Determining Geometry Once the Lewis structure of a molecule or ion is determined the 3-D shape of the molecule can be determined. So the steric number of Si is 4. So the steric number of Si is 4.
Postby An Dang 3F Mon Nov 20 2017 340 am. Sif4 lewis structure how to draw the lewis dot structures of covalent compound yeah chemistry. The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair.
Under prolonged exposure to heat the containers may rupture violently and rocket. By drawing the Lewis Structure you will see that the most stable structure is OSiO. Since SiO2 has two places of.
Sulfur Tetrafluoride has 34 valence electrons out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. Lewis Structure ame Molecular geometry Name Molecular geometry Name Di Molecular geometry Name Molecular geometry Name Molecular geometry Name Molecular geometry Lewis cture. Compound Draw Lewis Structure Polar or Nonpolar Electron Groups around central atom of Bonded Groups around central atom of Lone Pairs around the central atom Electron Molecular Geometry Geometry of the shape or compound compound the SiF4 CSez CICP BBrH2 CF2S SeO2 PI3.
CH4 Valence electrons 18 N03- Valence electrons Lewis. Silicon tetrafluoride appears as a colorless nonflammable corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. What is the molecular geometry of SiF4.
HCN Valence electrons 17. SiF4 Lewis Structure Molecular Geometry Hybridization and Polarity SIF4 is a covalent compound which consists of silicon and fluorine atoms. It has a molecular geometry of the formula AX4E.
It is named tetrafluorosilane or silicon tetrafluoride. Is the molecule polar. SiF4 Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar.
Youll have a pair of electrons left over after filling octets of the F atoms. It is a well-known haloalkane or halomethane having a high bond strength between the carbon and fluorine atoms becoming quite a stable compound. Since there are seven Fluorine F atoms it will be necessary.
To find out its Molecular Geometry we first examine its Lewis structure to know the arrangement of. In SiF4 the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. What types of bonds does the molecule have.
Also Know does silicon tetrafluoride have a dipole moment. SiF4 Valence electrons 14. Draw the lewis structure for COFH.
Is the molecule polar. Hence Si atom is sp3 hybridized in this compound and SiF4 gets tetrahedral molecular geometry. CF4 Lewis Structure Molecular Geometry Hybridization and Polarity.
- ppt video online download. Molecular Geometry Complete the table. SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons.
Electron and molecular geometry illustration Electron And Molecular Geometry.
Chem Molecular Shape Molecular Geometry Scientific Tutor
7 10 Notes Shapes For Covalent Structures Ppt Download

For Sif4 Draw The Lewis Structure Predict The Shape And Determine If The Molecule Is Polar Or Nonpolar Study Com
Chem Molecular Shape Molecular Geometry Scientific Tutor
What Is The Molecular Geometry Of Sif4 Quora

Sif4 Molecular Geometry Bond Angles Electron Geometry Youtube

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
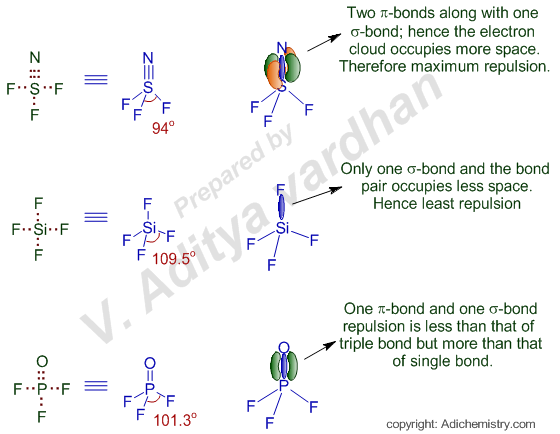
Vsepr Theory Bond Angles Nsf3 Sif4 Pof3

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Determine The Electron Geometry Eg And Molecular Geometry Clutch Prep

Silicon Tetrafluoride Sif4 Lewis Dot Structure Youtube

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube

Sf4 Molecular Geometry Shape Youtube