What Is The Electron Geometry Of Ch2o
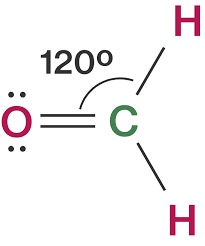
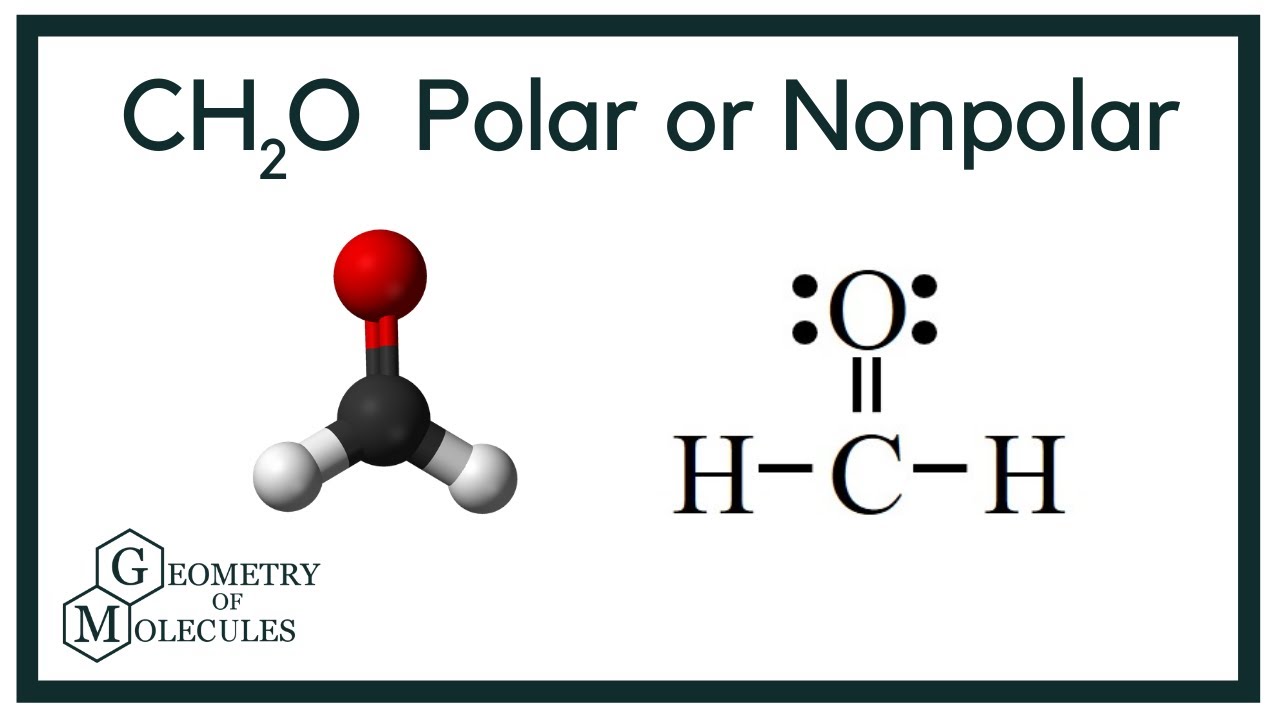
Both the electron pair geometry and the molecular geometry are PLANAR TRIGONAL. The molecular geometry of CH 2 O is trigonal planar with asymmetric charge distribution.
Ch2o Molecular Geometry Polarity Bond Angle Shape Geometry Of Molecules
We must first draw the Lewis structure for CH₂O.
What is the electron geometry of ch2o. What is the electron geometry of ICl5. The electron-domain geometry of PF6 is Octahedral since the central atom Phosphorus has an electron pair geometry which is octahedral Molecular geometry for CH2O. Electron geometry for this is tetrahedral.
Total valence electron of CH2O Valence electrons of Carbon Valence electrons of Oxygen Valence electrons of Hydrogen 4621 12 valence electrons of CH2O Thus CH2O has a total of twelve valence electrons that can help in drawing its Lewis structure. Here the octets of both Carbon and Hydrogen are. Thus in the Lewis structure of CH2O the central Carbon atom forms two single bonds with two Hydrogen atoms and one double bond with an Oxygen atom.
What is the electron geometry of ClF3. There are two lone pairs around oxygen which make up the last two electron groups. There are two O-F single bonds which makes 2 electron groups.
Therefore this molecule is polar. Here the octets of both Carbon and Hydrogen are completed and only Oxygen has two lone pairs of electrons. S p 2 hybridizationthe FOURTH electron on carbon is conceived to participate in a π bond with an electron from the oxygen atom to form an unsaturated C O ie.
CH2O - Formaldehyde. First draw the Lewis dot structure. Draw the most appropriate Lewis structures for CH2O.
Is not electronic and molecular geometry with respect to carbon trigonal planar The carbon shares 3 electrons with electrons from carbon and oxygen to form 2 C H and 1 C O bonds. In formaldehyde the central atom has three electron clouds emanating from it. The molecular geometry shape of CH2O is _____.
Types of Chemical Reactions Review. First draw the Lewis dot structure. What is the Lewis structure of CH2O.
Moreover the Valence Shell Electron Pair Repulsion VSEPR theory says the molecular geometry of a molecule is trigonal planar if the bond angle is 120 or nearer to it. First we need to calculate the to view the full answer. How many o and π bonds are there.
S p 2 hybridizationthe FOURTH electron on carbon is conceived to participate in a π bond with an electron from the oxygen atom to form an unsaturated C O ie. What is the electron geometry of SF6. What is the electron geometry of BrF5.
What is the hybridization of the central atom. What is the electron-pair geometry for C in CH2O. THIS SET IS OFTEN IN FOLDERS WITH.
The carbon shares 3 electrons with electrons from carbon and oxygen to form 2 C H and 1 C O bonds. Thus in the Lewis structure of CH2O the central Carbon atom forms two single bonds with two Hydrogen atoms and one double bond with an Oxygen atom. The VSEPR model states that the electron regions around an atom spread out to make each one as far from the others as possible.
CH 2 O has a molecular geometry of AX3 trigonal planar shape and an sp2 hybridization. What is the electron geometry of SF4. 100 5 ratings Answer We are given molecule CH2O and we need to draw the molecular geometry of the molecule CH2O and find out the both electronic as well as molecular geometry.
The molecular geometry shape of CH2O is _____ Octahedral. Chemistry - Geometric Shapes of Molecule. A The electron-pair geometry for the ammonia molecule is tetrahedral with one lone pair and three single bonds.
The molecular geometry shape of BeH2 is _____. Formaldehyde has two lone pairs of electrons on the Oxygen atom and no lone pairs on the central atom. It is a trigonal planar in shape with bond angles of 120 degrees.
It is polar due to the difference in the partial charges on Carbon and Oxygen atom. What is the electron domain geometry and the molecular geometry. The CH2O is a tetra atomic molecule where the bond angles for the hydrogen-carbon-hydrogen H-C-H and hydrogen-carbon-oxygen H-C-O are 116 and 122 and the structure is bent shaped.
There are three electron regions around the central carbon atom. Then draw the 3D molecular structure using VSEPR rules. Electron Geometry BONDING shape of all electron groups Total of of of Molecular Geometry shape of just atoms around central atom Bond Species ELECTRON LONE Angles groups PAIRS groups CC4 H2S HCN CH2O NCI3 CO2 SO2 NO.
Fill in the blank 4 There are fill in the blank 5 lone pairs around the central atom so the molecular geometry shape of CH2O is fill in the blank 6. Formaldehyde Lewis Structure CH2O Electron geometry. The molecular geometry shape of NF3 is _____.
Does the molecule have a dipole.

Ch2o Lewis Structure Molecular Geometry And Hybridization Techiescientist

Ch2o Molecular Geometry Polarity Bond Angle Shape Geometry Of Molecules

Ch2o Lewis Structure Valence Electrons Hybridization Geometry Of Molecules

What Is The Molecular Geometry Of Ch2o Quora
Ch2o Molecular Geometry Polarity Bond Angle Shape Geometry Of Molecules

Ch2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
Vsepr Theory And Molecular Geometry Bf3 Ch2o Hcn Becl2 Ch2cl2 Socl2 So2 Youtube

Ch2o Lewis Structure Molecular Geometry And Hybridization Techiescientist
Ch2o Molecular Geometry Shape And Bond Angles Youtube

Ch2o Molecular Geometry Shape And Bond Angles Formaldehyde Youtube

Ch2o Lewis Structure Molecular Geometry And Hybridization Techiescientist

7 Draw The Most Appropriate Lewis Structure S For Ch2o Wh Clutch Prep
Ch2o Lewis Structure Valence Electrons Hybridization Geometry Of Molecules

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube
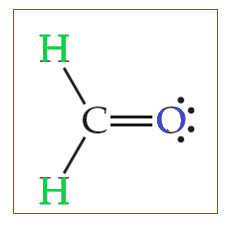
7 Draw The Most Appropriate Lewis Structure S For Ch2o Wh Clutch Prep
Https Nanopdf Com Download Molecular Shape And Polarity 5ae44375400d4 Pdf
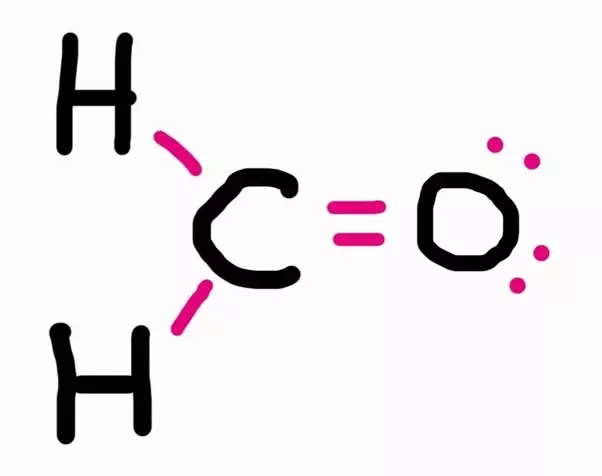
What Is The Electron Geometry For Ch2o Quora

Ch2o Molecular Geometry Shape And Bond Angles Youtube
