Cn- Lewis Structure Formal Charge
Valence electron of carbon. Draw Lewis structures for these ions and show which atom in each bears the formal charge.

In The Cyanide Ion Cn What Is The Formal Charge Of Nitrogen Study Com
Lets illustrate these rules by calculating the formal charges on the C and N atoms in the cyanide ion CN - which has the Lewis structure.

Cn- lewis structure formal charge. For Carbon Formal Charge 4 056 2 -1. What is the formal charge in CN- lewiss structure and how to calculate it. In the previous video we saw some steps for drawing dot structures in this video were going to use those same steps to draw a few more dot structures but were also going to talk about how formal charge relates to dot structures so well get back to this definition in a minute for right now lets draw a quick dot structure for the ammonium cation so NH 4 plus the first thing you do is find.
V a l e n c e e n o. Formal Charge Practice Problems Q. To find formal charges in a Lewis structure for each atom you should count how many electrons it owns.
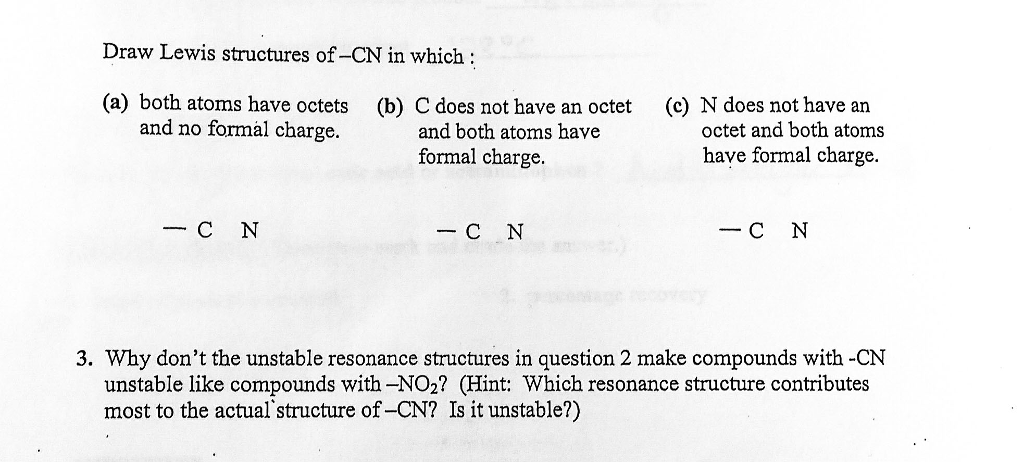
Draw a Lewis structure for SO2 in which all atoms have a formal charge of zero. Draw Lewis structure for CN CN and CN- and include formal charges lone pairs and nonbonding electrons. CN- Lewis Structure The Lewis structure of any molecule is a pictorial representation of the arrangement of atoms and electrons in the structure along with the bonds formed.
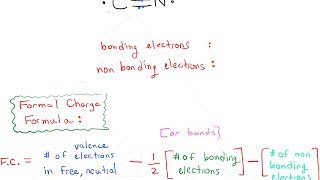
Determining formal charge on an atom. The formal charge of an atom equals the number of valence electrons in the isolated atom minus the number of electrons assigned to the atom in the Lewis structure. All Chemistry Practice Problems Lewis Dot Structure.
Now the formal charge can be calculated on each atom using the formula. Determine the number of valence electrons in sulfuric acid H₂SO₄ and then draw the corresponding Lewis structure with minimized formal charges. Also the net formal charge value -1.
So in Sals cyanide example the carbon would have a -1 formal charge and so we write that as a negative sign in a small circle next to the carbon. The formal charge FC on each atom can be calculated using the formula. A formal charge counts the number of electrons around an atom when it is bonded to other atoms in a molecule and is compared to the valence electrons of the same atom in isolation.
N in CN-C in CN. In order to calculate the formal charges for CN- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec. Count all of its lone pair electrons and half of its bonding electrons.
Draw Lewis structure for CN CN and CN- andinclude formal charges lone pairs and nonbonding electrons. To calculate the formal charge in CN- lewis structure. Since the elements are present in their least possible formal charge values we have got our most suitable Lewis Structure sketch for CN.
Formal charge Valence electrons unbonded electrons 12 bonded electrons First calculate the formal charge on carbon. How many resonance structures can be drawn for the dehydrogen antimonate ion h2sbo4 - in. Formal charge number of valence electrons owned by the isolated atom - number of valence electrons owned by the bound atom.
F C n o. Draw Lewis structures for ClNO and CN- then calculate the formal charges on each atom. N o n b o n d e d e n o.
From Lewiss structure it is clear that the number of bonds are 3 and each atom has one lone pair. For Nitrogen Formal Charge 5 056 2 0. The difference between the atoms number of valence electrons and the number it owns is the formal charge.
Formal charge tells you the charge of individual atoms in an ion neutral molecules too. The formal charge on an atom is calculated as the number of valence electrons owned by the isolated atom minus the number of valence electrons owned by the bound atom in the molecule. Match each of the atoms below to their formal charges.
The electrons that form bonds with the atoms are called bonding pairs of electrons. Draw Lewis structure for CN CN and CN- andinclude formal charges lone pairs and nonbonding electrons.

Cn Lewis Structure Cyanide Youtube

Draw Lewis Structure For Cn Cn And Cn And Include

Draw Lewis Structure For Cn Cn And Cn And Include Formal Charges Lone Pairs And Nonbonding Electrons

Lewis Resonance Structures Of Cn Method For Constructing Flickr

How To Calculate The Formal Charges For Cn Cynide Ion Youtube

Chapter 8 Basic Concepts Of Chemical Bonding Homework 1 8 11 12 13 16 18 19 20 22 24 25 30 31 32 35 37 39 40 42 43 45 47 49 Ppt Download

In The Best Lewis Structure For Cn What Is The Formal Charge On The N Atom A 0 B 1 C 1 D 2 Study Com

Formal Charge Chapter 8 Part 5 Youtube

Using Formal Charges To Evaluate Nonequivalent Resonance Structures Worked Example Video Khan Academy

Lewis Diagram Of The Cyanide Ion Worked Example Video Khan Academy

Formal Charge Problems 2 Cn Youtube
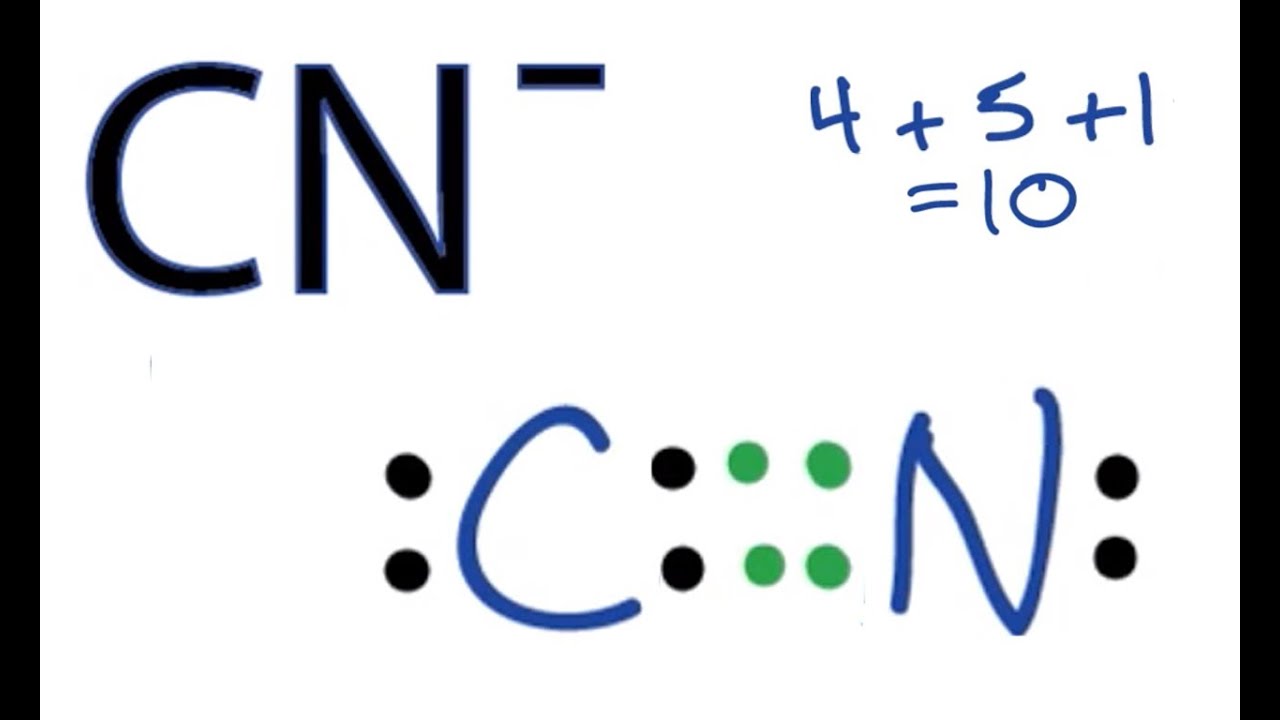
Cn Lewis Structure How To Draw The Dot Structure For Cn Chemical Bonding Success In Chemistry

How To Calculate The Formal Charges For Cn Cynide Ion Youtube
Draw Lewis Structures Of Cn In Which Both Atoms Chegg Com

Ap Chapter 8 Pr Chapter 4 Chemical Bonds

Cn Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Formal Charge Problems 2 Cn Youtube
