Ncl3 Molecular Geometry
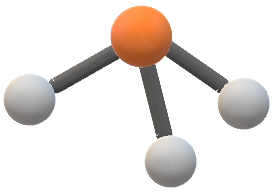
The molecular geometry of the molecule will be trigonal pyramidal. Emulates the 3d structure of various.

Homework 10 30 Points 1 Draw The Lewis Structure Chegg Com
Valence electrons of Phosphorus Valence electrons of Chlorine.

Ncl3 molecular geometry. Asked Sep 20 2016 in Chemistry by SarahC. What is the molecular geometry of sncl3. The ring structure of NCl3 is similar to that of 3-dimethyl-1-butanol.
The electron geometry of this molecule will be tetrahedral and it will have eqrmsrmprm3 eq hybridization. The net dipole moment of Nitrogen trichloride is 06 D. Learn how to draw a Lewis Structure and how to use it to assign a molecules shape geometry.
Use VSEPR theory to predict the molecular geometry of nitrogen trichloride NCl3. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. Lewis structure of Nitrogen trichloride contains 1 lone pair and 3 bonded pairs.
Hence the Geometry of the molecule of NCl3 is Trigonal pyramidal. Download Molecular Geometry For Ncl3 PNG. Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply this number by 3.
4 What is SP3 CH. According to the VSEPR theory any molecule that has three regions of electron density with no lone pair on the central atom always forms a trigonal planar geometry whereas a molecule that has three regions of electron density with the presence of lone pair on the central atom always forms a trigonal pyramidal geometry. What is the molecular geometry of NCl3.
LiI and NiO DrBob222 you said you would choose the answer d and I should know how. Log in Sign up. See full answer below.
Since the AlCl3 molecule doesnt contain any lone pair on the central atom and has a three region of electron density hence it forms a trigonal planar geometry. SOLUTION a The Lewis structure for the SnCl3-. This forces the two CH2 planes to be perpendicular.
Molecular geometry for ncl3 Home Science Math and Arithmetic History Literature and Language Technology Health Law Legal Issues Business Finance All Topics Random Leaderboard Related Topics Science Elements and Compounds Atoms and Atomic Structure Physics Trigonal pyramidal Is CH2 sp3 hybridized. 6 Can oxygen be sp3 hybridized. What Is The GEOMETRY For This Structure.
The electron-domain arrangement which does include any lone pairs attached to. 5 Are alkanes sp3. Total number of valence electrons of PCl3.
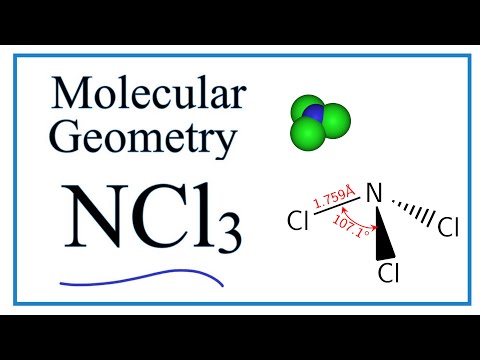
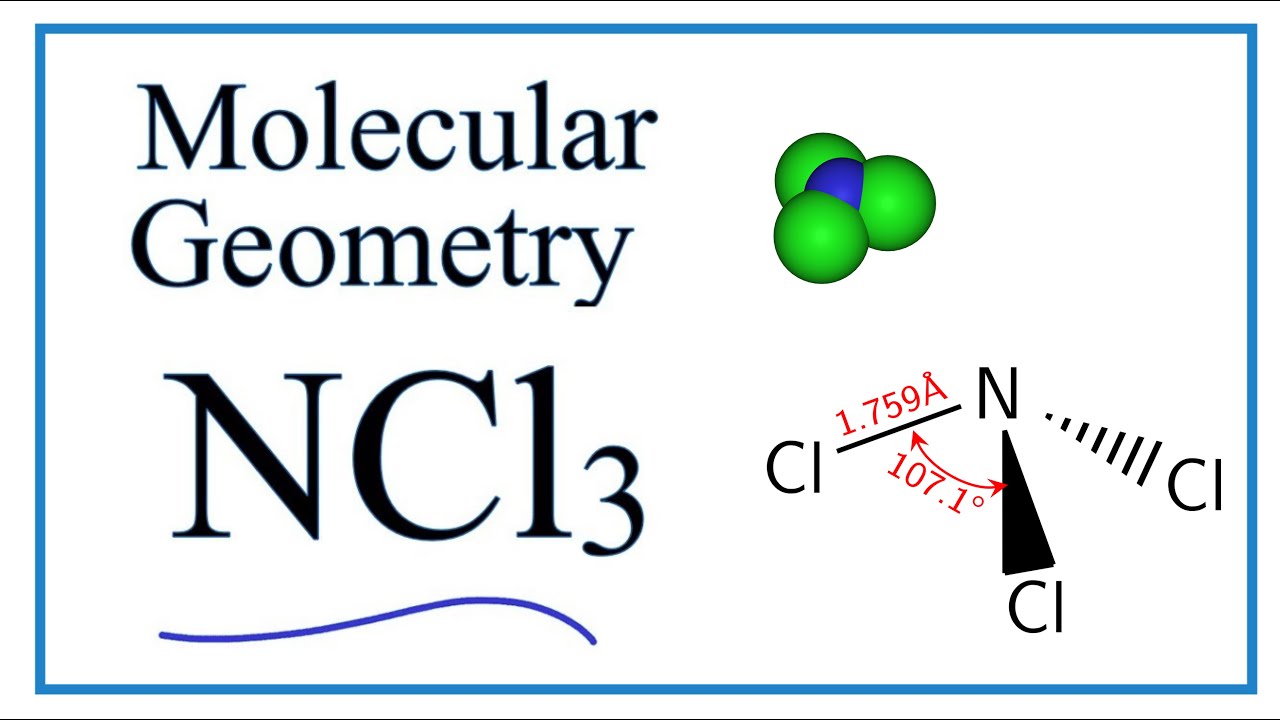
The N-Cl distances are 176 Å and the Cl-N-Cl angles are 107. Hence the Structure of the molecule of NCl3 is Tetrahedral since a lone pair of electron can be considered to be a bond pair. What is the molecular geometry of sf5 -.
The p orbitals used to form each π bond must be perpendicular to each other. The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral. Free unlimited access for 30 days limited time only.
NCl3 molecular geometry consists of a trigonal pyramid structure. Is CH2 sp3 hybridized. Favorite Answer First you need to know the structure of the molecule.
The center C atom is sp hybridized and is involved in two π bonds. Answered Sep 20 2016 by ThorXL. Determine the electron geometry eg and molecular geometry mg of NCl3.
3 Is ncl3 sp2 hybridized. NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a coloration proportional to the amount of NCl3 from the sampled indoor swimming pool air. 8 What is another word for hybridization.
The ring structure of NCl3. When we consider both lone pairs and bond pairs we are referring to the Structure of the molecule. The IM was validated in terms of.
What is the molecular geometry of ncl3. The molecular geometry of NCl3 is a p-type symmetry group consisting of four six-membered rings. Nitrogen trichloride is a yellow oily liquid with its pungent odor.
A linear b trigonal planar c bent d tetrahedron e trigonal pyramid. To do so we first need to draw a Lewis structure for NCl 3. A eg tetrahedral mg tetrahedral B eg linear mg trigonal planar C eg trigonal planar mg bent D eg linear mg linear E eg tetrahedral mg trigonal pyramidal.
The other 3 C atoms are sp3 hybridized. NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a. The rings are p-type symmetry groups and they are connected by a cis-acting bond to the ring carbon.
Thus the electron-pair geometry is tetrahedral with three of. Molecular shape of NCl3. Determine the electron geometry eg and molecular geometry mg of ncl3.
The center C atom is sp hybridized and is involved in two π bonds. NCl3 molecular geometry consists of a trigonal pyramid structure. 5 73.
The central Sn atom is surrounded by one nonbonding electron pair and three single bonds. The Correct Answer is. What is the molecular geometry of ncl3.
An NCl3 molecule would be a trigonal pyramidal because it has one center N atom with 3 Cl surrounding it but also a. Our sampling of the monitored swimming pool environments evidenced a mean NCl3 level 637-220 ugcu m higher than the recommended WHO value 500 ugcu m. The three atoms of chlorine bond with the nitrogen atom by a single bond ie.
NCl3 Lewis Structure and Molecular Geometry Geometry - YouTube. Get the detailed answer. 7 Why is ammonia sp3 hybridized.

Determine The Electron Geometry Eg And M Clutch Prep

File Ncl3 Dimensions Svg Wikimedia Commons

Draw The Lewis Structure For Ncl3 And Provide The Following Information A Number Of Electron Groups B Electron Pair Geometry C Bond Angle D Number Of Bonded Electrons E Molecular Geometry F
Wn Ncl2 Lewis Dot Structure Molecular Geometry Bond Angle

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube
Warm Up Draw Lewis Structures For The Compounds Below Cf4 Bf3 Co2 Ppt Download

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

Ncl3 Molecular Geometry Bond Angles Electron Geometry Nitrogen Trichloride Youtube
Solved What Is The Shape Around The Indicated Atom In Each Molecule Course Hero
Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Is Ncl3 Polar Or Nonpolar Techiescientist

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
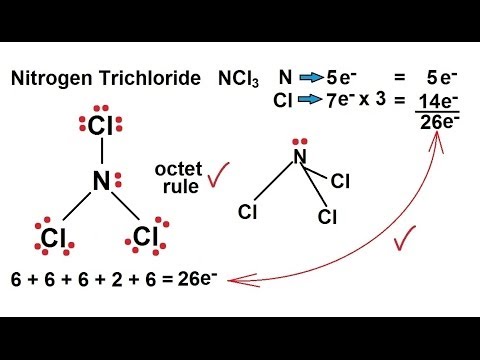
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

The Total Number Of Lone Pairs In Ncl3 Is Study Com

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

Determine The Electron Geometry Eg And M Clutch Prep
What Is The Molecular Geometry Of Ncl3 Quora