Hypochlorous Acid Lewis Structure
Please include all nonbonding electrons. The next thing to do is to insert pairs of.

Hclo Definition Lewis Structure Video Lesson Transcript Study Com
Draw two possible Lewis structures for hypochlorous acid with the atoms arranged as written HOCl and HCIO and then use formal charges to predict which of the two orders is preferred.

Hypochlorous acid lewis structure. This means that even though Oxygen atom is more electronegative than Chlorine it will still be placed at the center of the HOCl Lewis structure. Hypochlorous acid is a chlorine oxoacid with formula HOCl. A step-by-step explanation of how to draw the HOCl Lewis Structure Hypochlorous AcidBecause HOCl is an acid well put the Hydrogen atom on the outside of.
HOCl HClO HOCl is the preferred arrangement. While most Lewis structures are drawn with the most electronegative element in the center hypochlorous acid is more stable when oxygen is in the center since H O and Cl all have a FC of 0. Four are used as bonding electrons and the remaining 10.
Hypochlorous acid has a partial dissociation in water because it is a weak acid and its acid dissociation constant ka is. One with oxygen as the central atom and the other with chlorine as the center. The Lewis structure of hypochlorous acid has oxygen O with single bonds between hydrogen and chlorine.
Lets consider the lewis structure. Ap 2005 chemistry_b scoring guidelines - The College Board d In an experiment 2000 mL of 0175 M HOClaq is placed in a flask and titrated. ReadDownload File Report Abuse.
Which one is the central atom. Now we decide how hydrogen chlorine and oxygen are bonded together. HOCl is called Hypochlorous Acid.
It has a role as a human metabolite an EC 3117 acetylcholinesterase inhibitor and an EC 25118 glutathione transferase inhibitor. NH 4 lewis structure. It contains single bonds between the atoms.
A Draw a complete Lewis electron-dot structure for the CS2 molecule. Lewis Structure of Hypochlorous Acid In the Lewis structure we see that hypochlorous acid has 14 valence electrons. The bond angle in ClO- is 180º.
For the HOCl Lewis structure there are a total of 14 valence electrons. Steps of drawing the lewis structure of NH 4 are explained in this tutorial. Posted on March 05 2017.
HOCl Hypochlorous Acid Lewis Structure. The Lewis structure of hypochlorous acid has oxygen O with single bonds between hydrogen and chlorine. Lewis Structure Step 1.
Whats the Lewis structure for HOCl. In the case of polarity the OCl2 is a polar one as. It has double bonds.
There are no charges on atoms. Four are used as bonding electrons and the remaining 10 are nonbonding electrons on oxygen and chlorine. So chlorine atom is the center atom in HOCl molecule.
This means that even though Oxygen atom is more electronegative than Chlorine it will still be placed at the center of the HOCl Lewis structure. First we need to count the valence electrons of hydrogen H chlorine Cl and oxygen O. Hypochlorous Acid Lewis Dot Structure Free PDF eBooks.
It is a member of reactive oxygen species and a chlorine oxoacid. A weak unstable acid it is the active form of chlorine in water. The hybridization of chlorine and oxygen in the ClO- the molecule is Sp 3.
In the Lewis structure we see that hypochlorous acid has 14 valence electrons. The overall formal charge in ClO- is -1. The central atom is oxygen.
ClO- is a non-polar molecule as it has a symmetrical structure and zero dipole moment. Lewis structure of HOCl Hypochlorous Acid molecule contains O-H bond and O-Cl bond. Hypochlorous acid methyl ester.
Which of the following is NOT TRUE about hypochlorous acids Lewis structure. The total valence electron available for the Hypochlorite ClO- lewis structure is 14. Hypochlorous acid has two resonance structures.
There are three lone pairs on chlorine atom and two lone pairs on oxygen atom. From the Lewis structure of OCl2 it is clear that the oxygen molecule has two lone pairs of electrons which change the molecular geometry of the molecule from linear to bent or V-shaped. Moreover the hybridization of OCl2 is sp3 which means there exist four hybrid orbitals of similar energy that stabilizes the overall structure of the molecule.
In water hypochlorous acid occurs both as a.
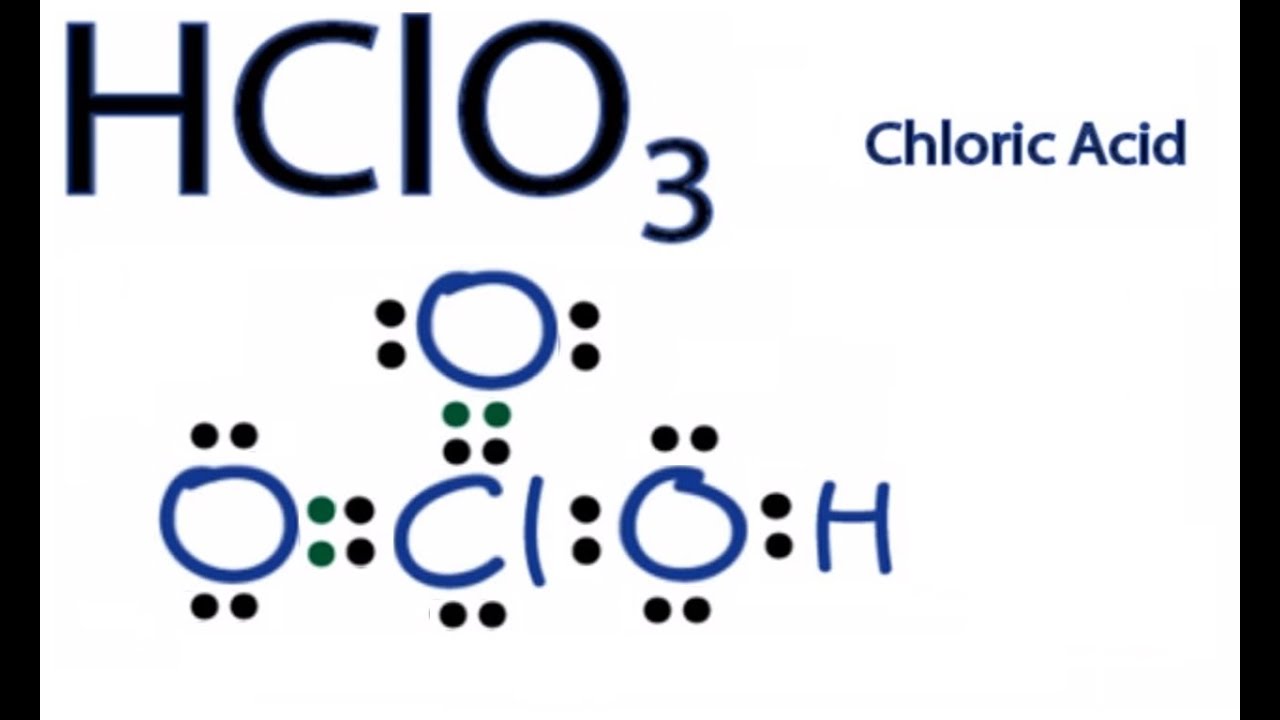
Hclo3 Lewis Structure How To Draw The Lewis Structure For Hclo3 Youtube

Hclo Definition Lewis Structure Video Lesson Transcript Study Com
Hypochlorous Acid Barium Salt Ba Clo 2 Pubchem

Dichlorine Monoxide Everything You Need To Know With Photos Videos

Hclo Definition Lewis Structure Video Lesson Transcript Study Com

Hypochlorite Png Images Pngwing

Draw The Lewis Structure With The Atoms Arranged As Hclo Include All Non Bonding Electronsand Brainly Com
Hypochlorous Acid Wtinternational
Hypochlorous Acid Hclo Pubchem
Hypochlorous Acid Wtinternational

Hypochlorous Acid Png Images Pngwing
Hypochlorous Acid Wtinternational
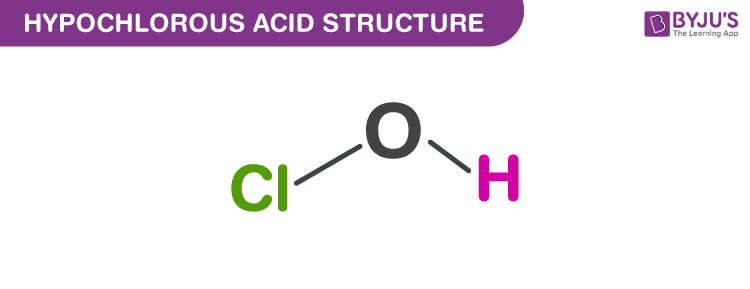
Hypochlorous Acid Structure Properties Uses Of Hocl

Covalent Bonding Formation Of Hydrogen Chloride Cl Cl H H Ppt Download

What Is The Lewis Structure For Hclo Chemistry Stack Exchange

Hocl Lewis Structure How To Draw The Lewis Structure For Hocl Youtube

What Is The Lewis Structure For Hclo Chemistry Stack Exchange
Why Is Hclo3 A Stronger Acid Than Hclo Quora


