Do Lewis Acids Affect Ph
HBr a Lewis Acid. Post by Brevin Hensley 1C Sun Dec 09 2018 913 am.

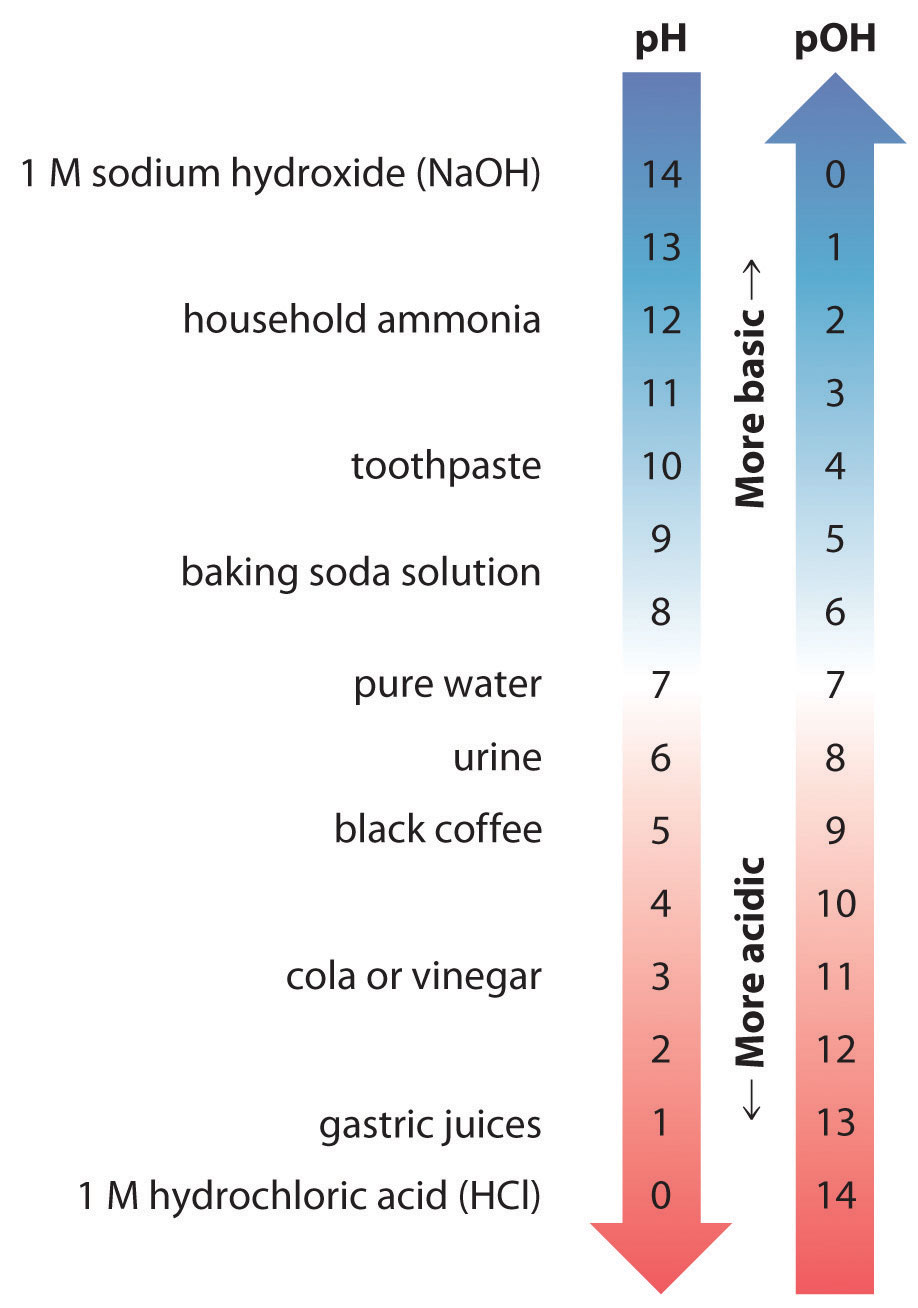
Let S Talk Science A Ph Solution Washington State Department Of Ecology
The Lewis acid-base theory doesnt affect the category of compounds we have called bases because any Brønsted base must have a pair of nonbonding electrons in order to accept a proton.
Do lewis acids affect ph. Because Lewis acids attract electron pairs Lewis acids are sometimes called electrophiles. Even some complex anions such as the sulfate anion SO 42- can donate pairs of electrons. Common Lewis acid catalysts are based on main group metals such as aluminum boron silicon and tin as well as many early and late d-block metals.
Brevin Hensley 1C Posts. Lewis bases will usually act as Bronsted bases as well and protonate in water thus raising the pH. For example titanium tetrachloride is a yellow liquid at room temperature.
Raising the pH yields hydroxide which is a much better nucleophile than water and results in nucleophilic. Effect of pHpH influences the acid dissociation and solubility of calcite that promotes reaction between the dissociated acid and calcium ions. For example protonating ammonia H NH3 NH4 lowers the pH of the solution because it produces a basic Lewis adduct.
An electron-pair donor such as the OH-ion. This theory however vastly expanded the family of compounds that can be called acids. H2O a Lewis Acid b Lewis Base c Neither.
The π-systems which are rich in electrons such as benzene ethyne and ethene exhibit great electron pair donating capabilities. Fri Sep 28 2018 725 am. Board index Chem 14A Acids and Bases Lewis Acids.
So to understand how Lewis acidbases affect pH. Using the Lewis concept of acids and bases identify the Lewis acidand basein each of the following reactions. In this video we cover the basics on the chemical makeup of acids bases salts and the pH levelAcids bases salts and ph levelAcids bases and salts are ino.
Lewis therefore argued that any substance that can act as an electron-pair donor is a Lewis base. Think of a proton H as a Lewis acid - it has a positive charge and could accept a pair of electrons. M whereas acidic solutions have pH 700 corresponding to H_3O 10 times 107 and basic solutions have pH 700 corresponding to H_3O 10 times 107.
Write the two equations necessary to make this determination. As already mentioned many Lewis acids are water reactive and will hydrolyze in water. In Lewis acid catalysis of organic reactions a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate.
Based on their observations calcite is positively charged almost in all ranges of pH. If we protonate hydroxide H OH H2O we. Several authors Lebell and Lindström 1982 Thompson and Pownall 1989 Schramm et al 1991 Legens et al 1998 studied the change of zeta potential on calcite surfaces.
Lewis acid-base complexes frequently have very different properties from the separate compounds from which they were formed. Lewis acid-base complexes frequently have very different properties from the separate compounds from which they were formed. When adding H to a solution we either raise or lower a pH.
Kcontinue to change pH and approach an endpoint this ratio changes more with each successive unit of pH change because almost all of the acid or conjugate base is consumed. Be sure to identify the product as a strong or weak acid or base. Because Lewis acids attract electron pairs Lewis acids are sometimes called electrophiles.
It is so Lewis acidic that it reacts with moisture in. How would the pH of a solution differ if you used a strong acid. Acids and Bases affecting the pH.
Then write the effect of cach reaction and the overall reaction. Examples of such anions include H and F. It is so Lewis acidic that it reacts with moisture in.
Acids and bases catalyze the hydrolysis of the phosphodiester bonds in the phosphate deoxyribose backbone. AlNO33 a Lewis Acid b Lewis Base c Neither. Goes into changing conjugate baseacid.
Acids and bases destroy the covalent bonds that hold together the base pairs in a strand of DNA. CH3NH2 a Lewis Acid b Lewis Base c Neither. Koxides of elements can be thought of as Lewis acids and bases based on their bond character with O and will make water acidic.
Hydrolysis is slow virtually nonexistent at neutral pH. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate such as oxygen. Recall also that the pH of a neutral solution is 700 H_3O 10 times 107.
EFFECTS OF SALTS ON PH Lewis Acids and Bases Determine the effect on the pH when copperll sulfate salt is added to water. For example titanium tetrachloride is a yellow liquid at room temperature. Acids and Bases affecting the pH.
Ketzbook solved acid-base practice problems and then explains in depth what pH is and relates it to other log scales in science. 2 posts Page 1 of 1.

Conjugation And Resonance In Organic Chemistry Organic Chemistry Chemistry Organic Chem

Appendix Science Chemistry Chemistry Classroom Study Chemistry

Effect Of Ph On The Product Yield Download Scientific Diagram
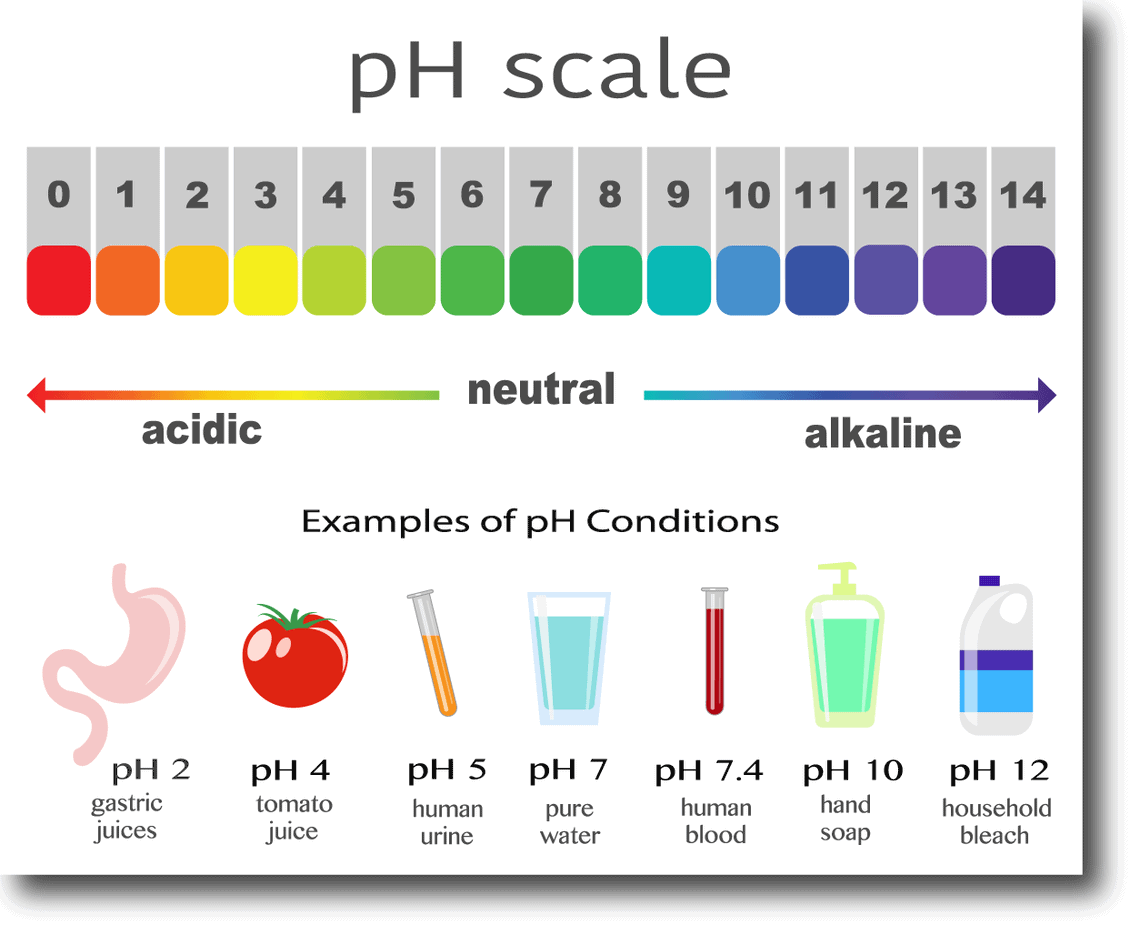
What Is Oral Ph And How Does It Affect Your Health Healthy Living Wilmington Holistic Dentist All About Smiles

The Ph Scale Biology For Majors I
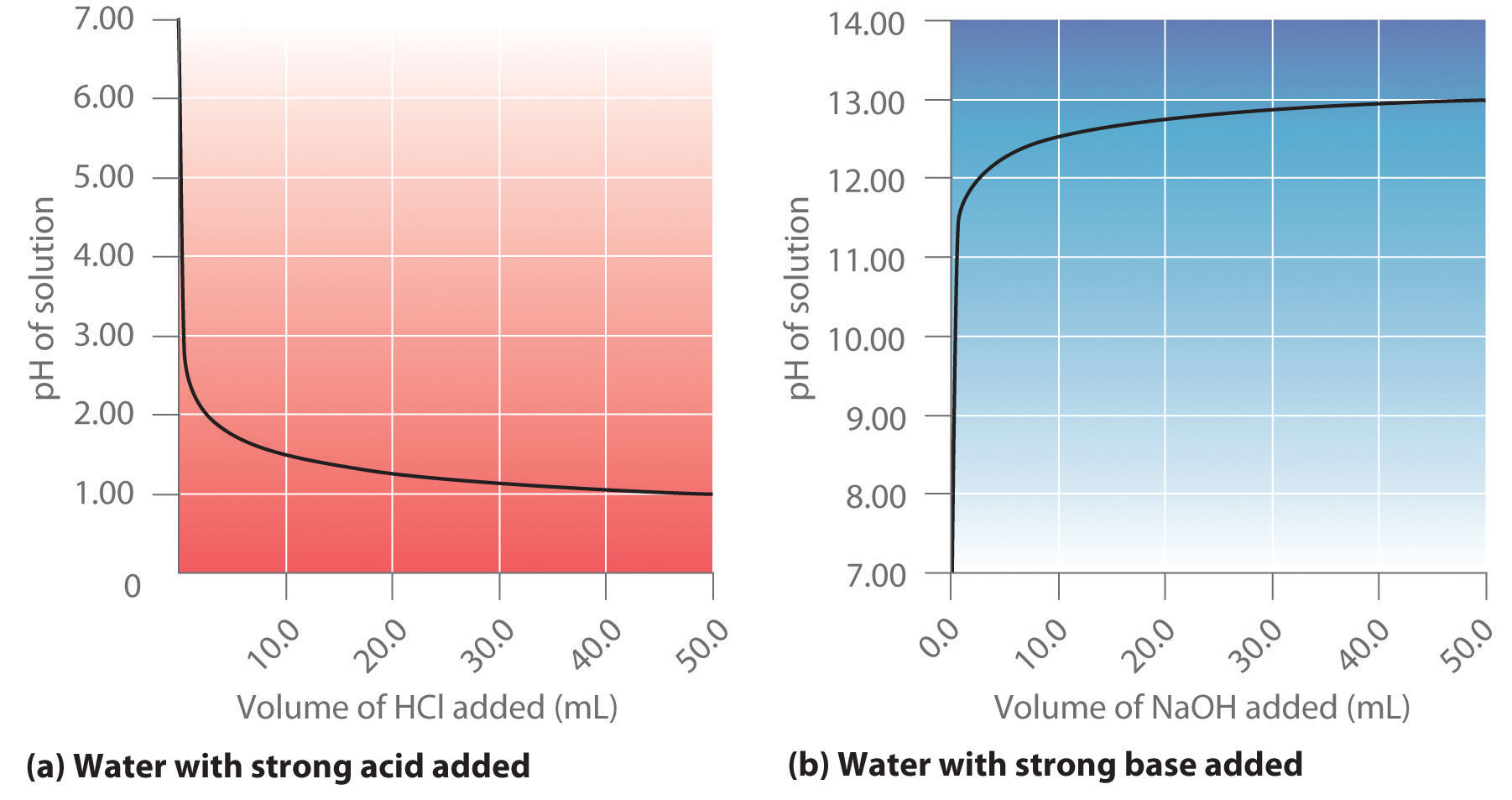
17 3 Acid Base Titrations Chemistry Libretexts
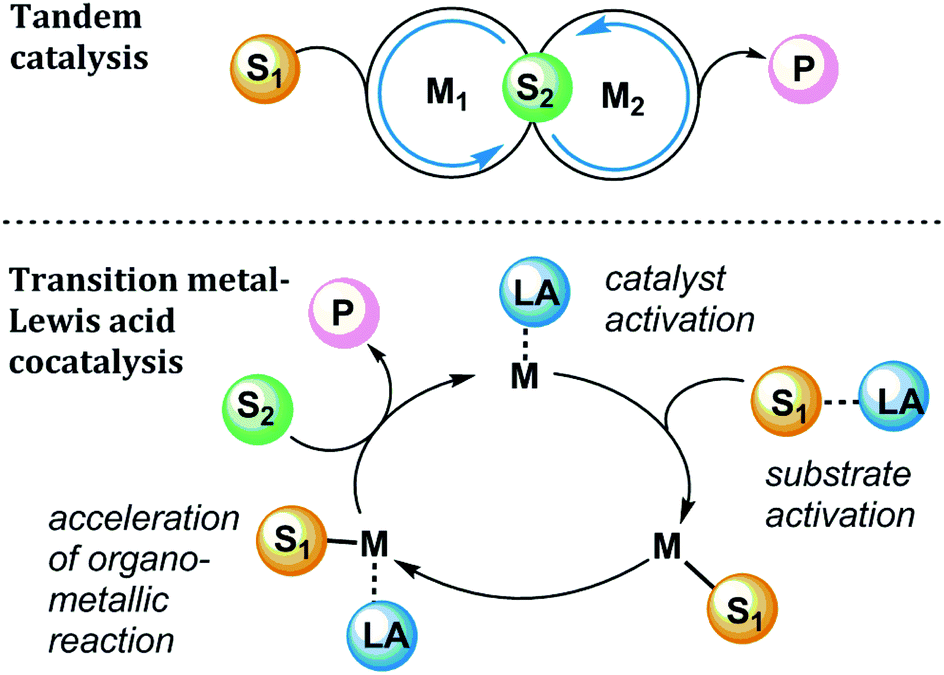
The Roles Of Lewis Acidic Additives In Organotransition Metal Catalysis Organic Biomolecular Chemistry Rsc Publishing Doi 10 1039 C8ob02856g

Just In Time Science Teaching Resources Chemistry Activities Chemistry Lessons
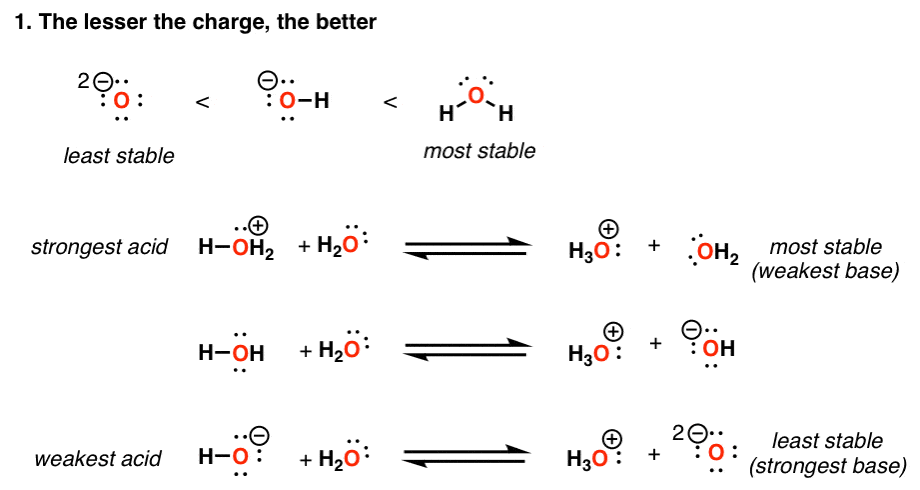
Acidity Trends In Organic Chemistry Master Organic Chemistry
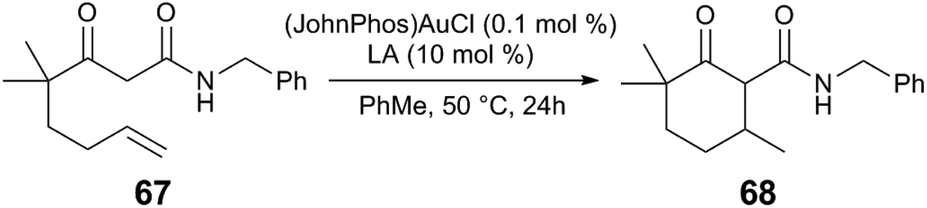
The Roles Of Lewis Acidic Additives In Organotransition Metal Catalysis Organic Biomolecular Chemistry Rsc Publishing Doi 10 1039 C8ob02856g

Acid Catalysis An Overview Sciencedirect Topics

Did You Know These Facts About Acids And Bases

Image Result For Alkaline And Acidic Foods Alkaline Diet Ph Chart Alkaline Diet Recipes

Aqueous Acid Base Equilibriums


