Asf6- Lewis Structure Polar Or Nonpolar
Formal charge Valence electrons unbonded electrons 12 bonded electrons Now we will calculate the formal charge on carbon which is the central atom in the CF4 lewis structure. Answer AsF5 Arsenic pentafluoride is Nonpolar What is polar and non-polar.

Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples Covalent Bonding Chemical Bond Study Chemistry
AsF5 is a nonpolar molecule despite it has five As-F polar bonds.

Asf6- lewis structure polar or nonpolar. A polar molecule with two or more polar bonds must. To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures. SF6 is a nonpolar molecule.
Silicon tetrachloride is corrosive to tissue and metal. Nonpolar Lewis Structure Of Oxygen Gas Amazing Rust com Oxygen Gas Production C2f2 Lewis Structure Molecular Geometry alter playground Polarity Chemistry LibreTexts Is N2 Polar or Nonpolar. Sf6 Lewis Structure Polar Or Nonpolar.
It provides examples so you can quickly distinguish nonpolar molecul. Is sf6 polar or nonpolar. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. In AsF3 there are a total of 26 valence electrons present 21 from five fluorine atoms. It has a molar mass of 154954 gmol.
This is because the VSEPR theory says that when six fluorine atoms are arranged symmetrically around the sulfur atom the bond dipoles are cancelled. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. As a result it is a nonpolar molecule.
It is used to produce high-quality silica for commercial purposes. Is SF6 polar or nonpolar. SF6 is a nonpolar compound in nature because as per VSEPR theory six fluorine atoms are arranged symmetrically with the sulfur atom such that dipole moment of S-F bond gets canceled out making the SF6 a nonpolar compound.
When the electronegativities are not equal electrons are not shared equally and partial ionic charges develop. Academiaedu is a platform for academics to share research papers. Never really heard of.
Benzene is an organic compound with the molecular formula C6H6. In this tutorial we will discuss Selenium tetrafluoride SeF4 lewis structure molecular geometry polar or nonpolar hybridization bond angle etc. Selenium tetrafluoride is used to add fluorine to other chemical compounds or it acts as a fluorinating agent.
A polar molecule or ion is the one that has a net resultant dipole moment whereas a non-polar molecule or ion is the one that does not has a net resultant dipole moment. Due to its geometrical structure sef4 is polar. Draw the Lewis structure.
How to tell if a lewis structure is polar or nonpolar Depending on the relative electronegativities of the two atoms sharing electrons there may be partial transfer of electron density from one atom to the other. Use this equation given below. Notice that a tetrahedral molecule such as ceCCl_4 is nonpolar Figure PageIndex1.
Hence we can distribute 6 on each Cl and 2 per single bond for a total of 6622 16 putting the remaining 6 on. The greater the electronegativity difference the more ionic the bond is. Therefore both molecules are nonpolar however each bond is polar So we have 7771 22 valence electrons.
A stepbystep explanation of how to draw the asf6 lewis structure. Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. This video provides a fast way for you to determine if a molecule is polar or nonpolar.
List molecules polar and non polar. AlCl3 is a nonpolar molecule because its net dipole moment is zero and charges are uniformly distributed all over the atom. You would expect the molecule to be polar because of that unbonded pair.
High purity of silicon tetrachloride used in the manufacture of optical fibers. In this ScienceStruck post we provide you with the polarity and steps to create the Lewis dot diagram of this aromatic compound. Is sif4 polar or nonpolar.
Properties of Selenium tetrafluoride. Structure calculate total number. So Is AsF5 polar or nonpolar.
It provides examples so you can. AsF5 Polar or Nonpolar Detailed Explanation. This is because AsF5 has a trigonal bipyramidal molecular geometry which a symmetrical structure.
The total valence electron is available for drawing the Aluminium chloride AlCl3 lewis structure is 24. Lewis structure of a molecule helps in the figure out the molecular geometry bond structure hybridization boiling point melting point etc. Lewis Structure of AsF3.
To calculate the formal charge in CF4 lewis dot structure. Lewis structure is a pictorial representation of a molecule where each atoms valence electrons are placed according to the octet rule. A stepbystep explanation of how to write the lewis dot structure for sif4 silicon tetrafluoride.
Another non polar molecule shown below is. The induced charges due to As-F bonds get cancel and the molecule has a zero net charge. Polarity underlies a number of physical properties including surface.
The hybridization of the AlCl3 molecule is Sp 2 since it has a steric number equal to 3 that will form an Sp 2 hybrid. A stepbystep explanation of how to draw the hcch lewis structure ethyne. To know the polarity and other properties of any molecule it is vital first to understand its.
To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other. So is asf5 polar or nonpolar.
Also known as silicon tetrafluoride. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. In this article we will discuss Silicon tetrachloride SiCl4 lewis dot structure molecular geometry hybridization polar or nonpolar etc.

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
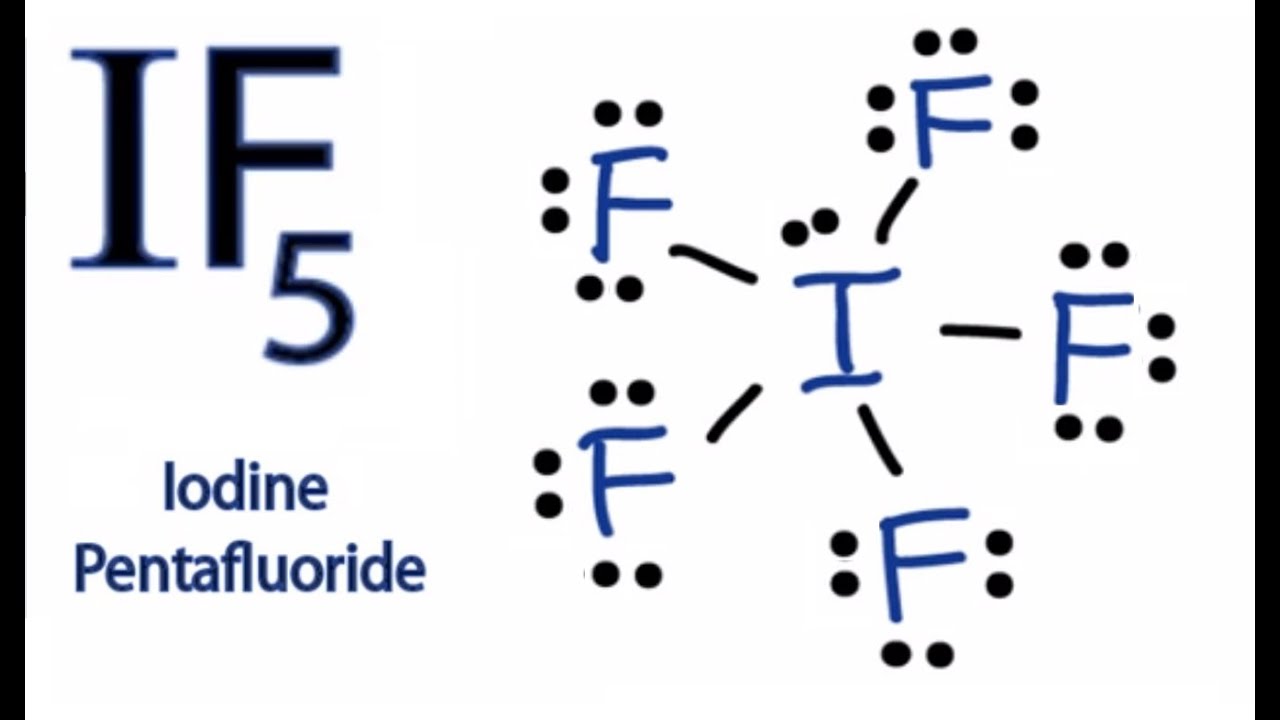
If5 Lewis Structure How To Draw The Lewis Structure For If5 Youtube

Clf5 Lewis Structure How To Draw The Lewis Structure For Clf5 Chlorine Pentafluoride Youtube

Pf6 Lewis Structure How To Draw The Lewis Structure For Hexafluorophosphate Youtube
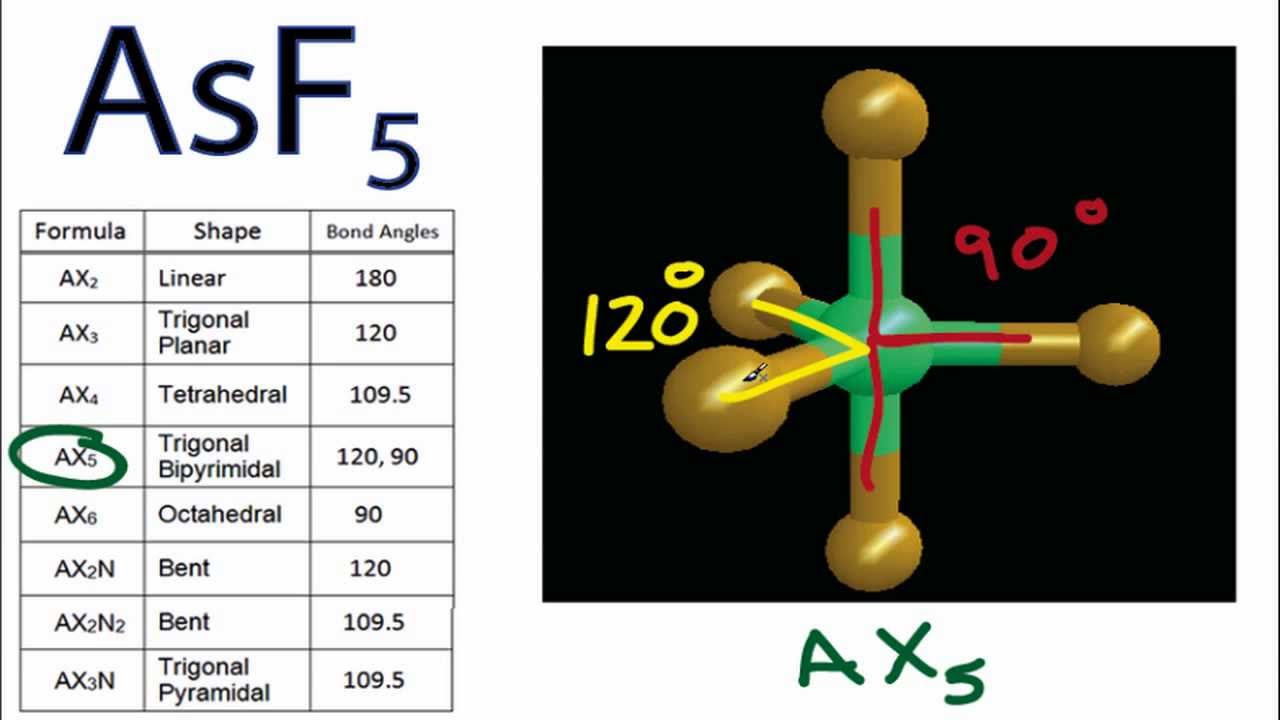
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Youtube

Is Sf6 Sulfur Hexafluoride Polar Or Non Polar Youtube

Asf6 Lewis Structure How To Draw The Lewis Structure For Arsenic Hexafluoride Ion Youtube

Polar And Non Polar Covalent Molecules Polar Vs Nonpolar Youtube Playlist Science Chemistry Molecules Polar
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
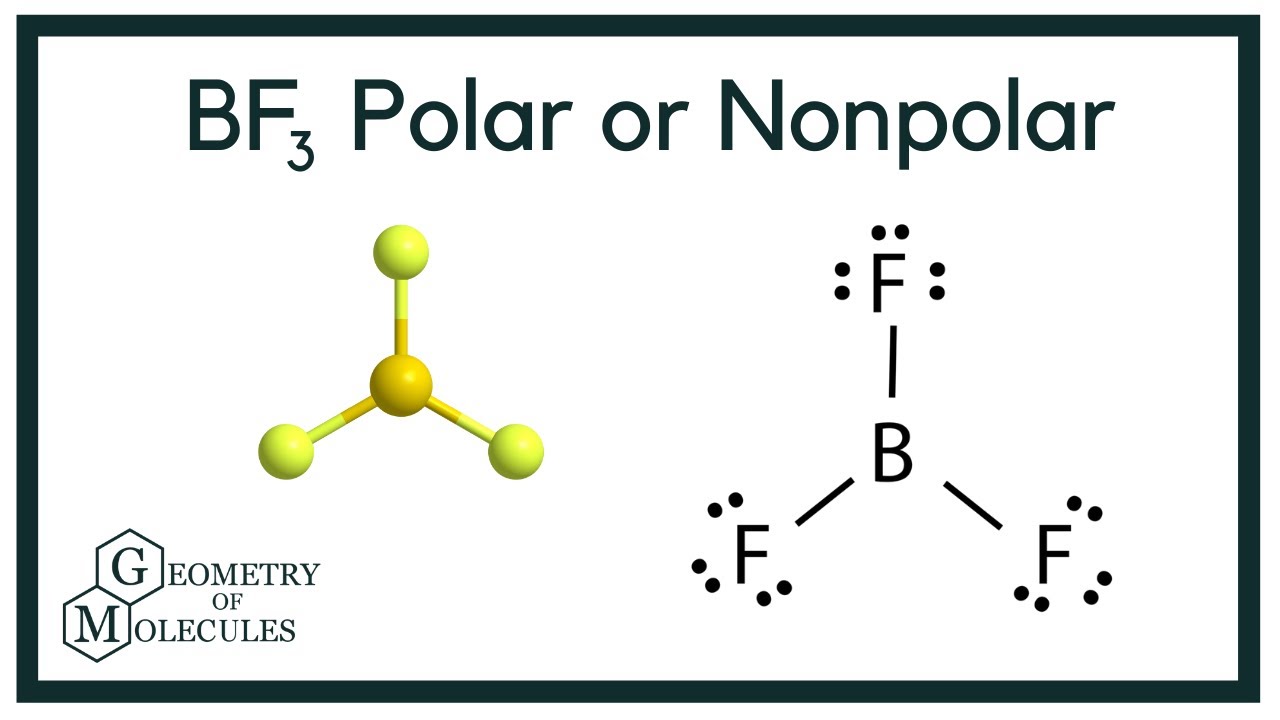
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles Molecular Geometry Molecular Bond

Science Coverage Is Hbr Polar Or Nonpolar In 2021 Electron Affinity Solubility Polar

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom
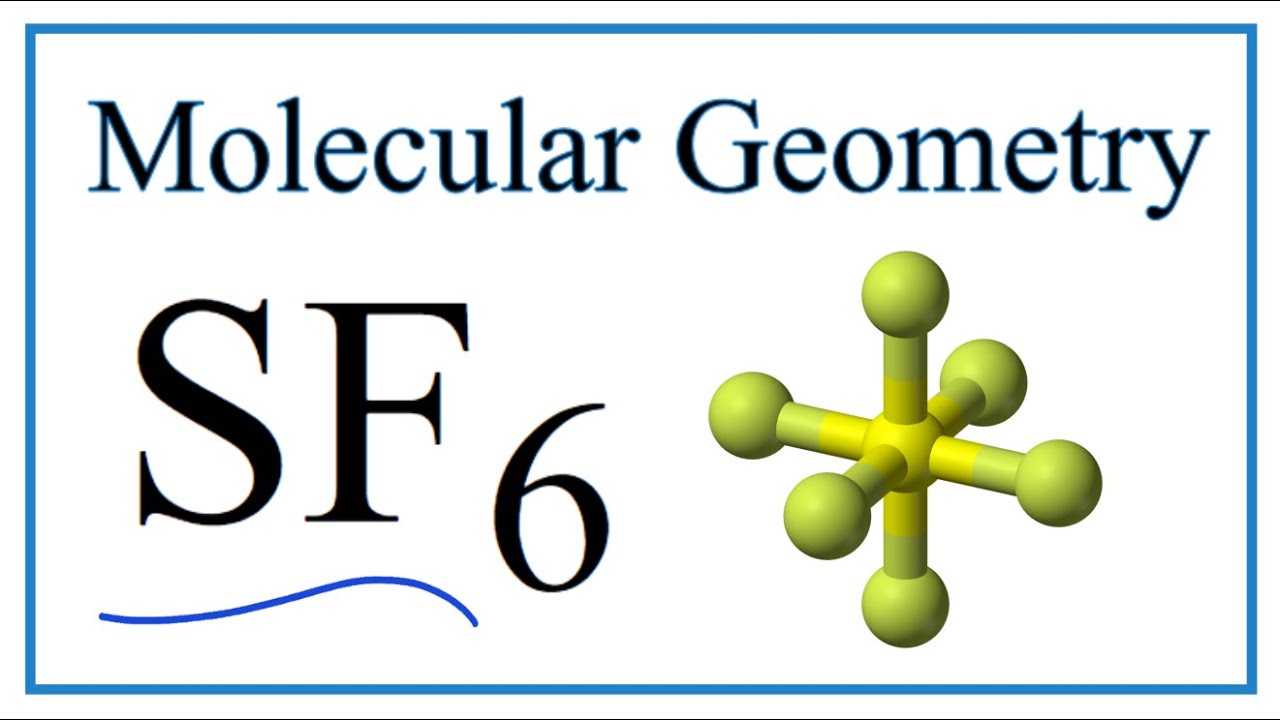
Sf6 Sulfur Hexafluoride Molecular Geometry Bond Angles Youtube
How To Tell If A Molecule Is Polar Or Non Polar Vsepr

Predict The Molecular Geometry Of Asf6 Arsenic Hexafluoride Youtube

