Brf3 Lewis Structure Electron Geometry
How many valence electrons does a molecule of BrF3 contain. Drawing BrF3 Lewis Structure is very easy to by using the following method.

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep
Steps to form BrF3 Lewis Structure.
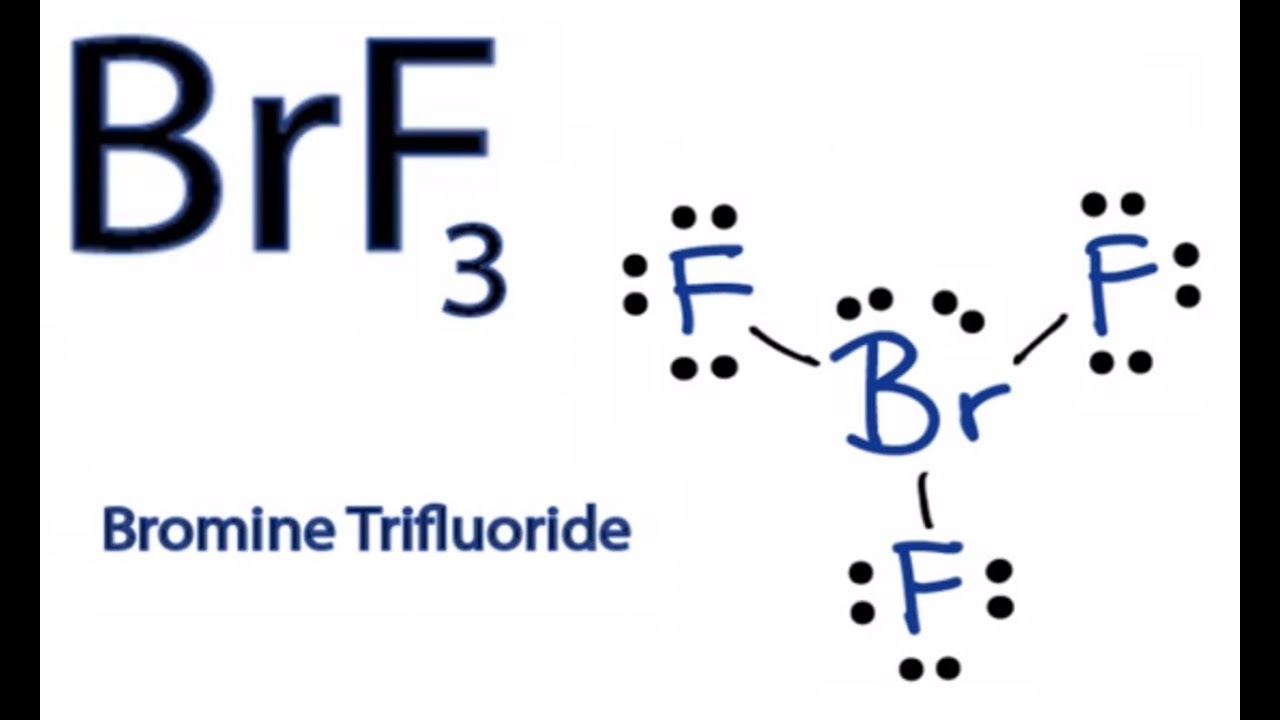
Brf3 lewis structure electron geometry. The total number of valence electrons in BrF3 7 73 7 21 28. Draw this VSEPR structure next to the Lewis structure. There are a total of 28 valence electrons for the BrF 3 Lewis structure.
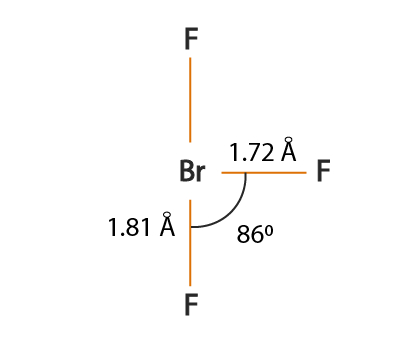
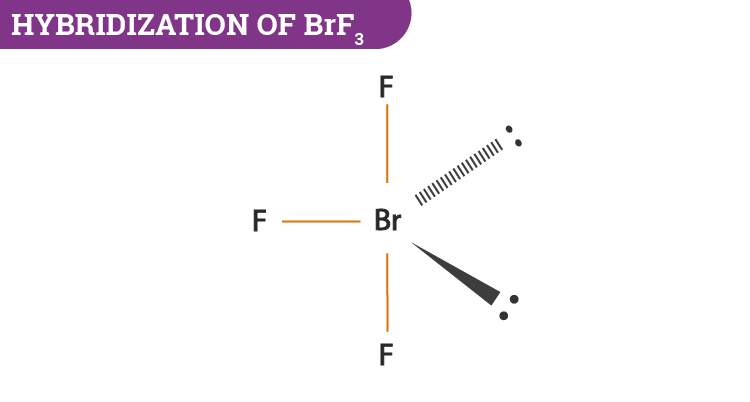
There are 3 atoms and 2 lone pair around the central atom Br of BrF3 which corresponds to AX3E2 or T-shaped The molecular shape fo BrF3 is D T-shaped. Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at the. BrF3 has a T-shaped or Trigonal Bipyramidal molecular geometry with a bond angle of 862 which is somewhat less than the typical 90.
When determining molecular geometry atoms and lone pairs are treated differently. The geometry of the CH3I molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CH3I geometrical shape in which the. BrF 3 contains three bonded and two nonbonded electron domains giving a trigonal pyramidal e-domain geometry and a T shaped molecular geometry.
Bromine trifluoride chemical formula is BrF3. There are a total of 28 valence electrons for the BrF 3 Lewis structure. The repulsion created by the electron pairs is higher than that of the Br-F bonds resulting in this angle.
BrF3 consists of seven electrons in its outermost shell. From the structure we can see that this molecule has a total of three bond pairs and two lone pairs. Thus the central atom is.
Therefore both of these elements will have a valency of 7. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets. Draw the Lewis structure for BrF3 b What is the electronic geometry of this molecule look at atoms and lone pairs.
BrF3 B r F 3 The structure of this given compound is shown below. So the hybridization of the BrF3 molecule is sp3d. What is the electron domain geometry and the molecular geometry.
Molecular Geometry of BF3. The bond angles are compressed relative tothose in a perfect trigonal bipyramid due to lone. Write the Electron Geometry Molecular Geometry Approximate bond angle Bond type ionic covalent polar covalent nonpolar Is BrF3 polar.
Does the molecule have a dipole. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. The chemical formula for Bromine Trifluoride is BrF3 and it reacts in different elements except for inert gases.
Both need 1 extra electron to complete their octets. Draw the most appropriate Lewis structures for BrF3. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle.
I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule. I2 I- - I3-.
The CH3I Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH3I molecule. The outer shell of the Bromine molecule has seven valence electrons and three of them bond with the three fluorine atoms. Sketch the Lewis structure of the molecule BrF3 showing in detail.
Hence its hybridization is sp3d. What is the hybridization of the central atom. The geometry of molecule of BF3 is Trigonal Planar With the reference of Chemistry Trigonal Planar is a model with three atoms around one atom in the middle.
After the bond formation it will further have two lone pairs and 3 BrF covalent bonds bonding pairs. As the hybridization value or the electron pair is equal to 5 it gives rise to sp3d hybrid orbitals. Br and F are both halogens belonging to group 7 in the periodic table.
Here in this post we described step. A the electron pairs on Br b molecular geometry c formal charge on Br d the polarity of the molecule polar or non-polar 2. The BrF3 molecular geometry is in T-shaped or Trigonal Bipyramidal with Bromine as the central atom.
How many o and π bonds are there. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. The shape of a molecule is based on its molecular geometry.
Oto Molecule Lewis Structure Formal Charge Electron Chegg Com
Page 5 Of 7 Formulas Pbrs Brf3 Tefs Molecular Chegg Com

Whats The Molecular Geometry Of Asf3 Ch3 Brf3 Clo3 Xef2 Bro2 Study Com
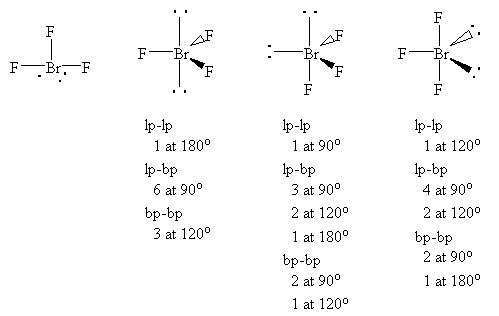
Brf3 Electron Geometry Shefalitayal

Brf3 Molecular Geometry Youtube
What Is The Hybridization Of Brf3 Quora

What Is The Molecular Shape Of Brf3 As Predicted By The Vsepr Theory Study Com

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
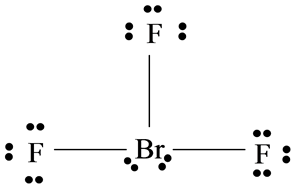
Solved Chapter 22 Problem 21e Solution Selected Solutions Manual General Chemistry 10th Edition Chegg Com
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride
Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
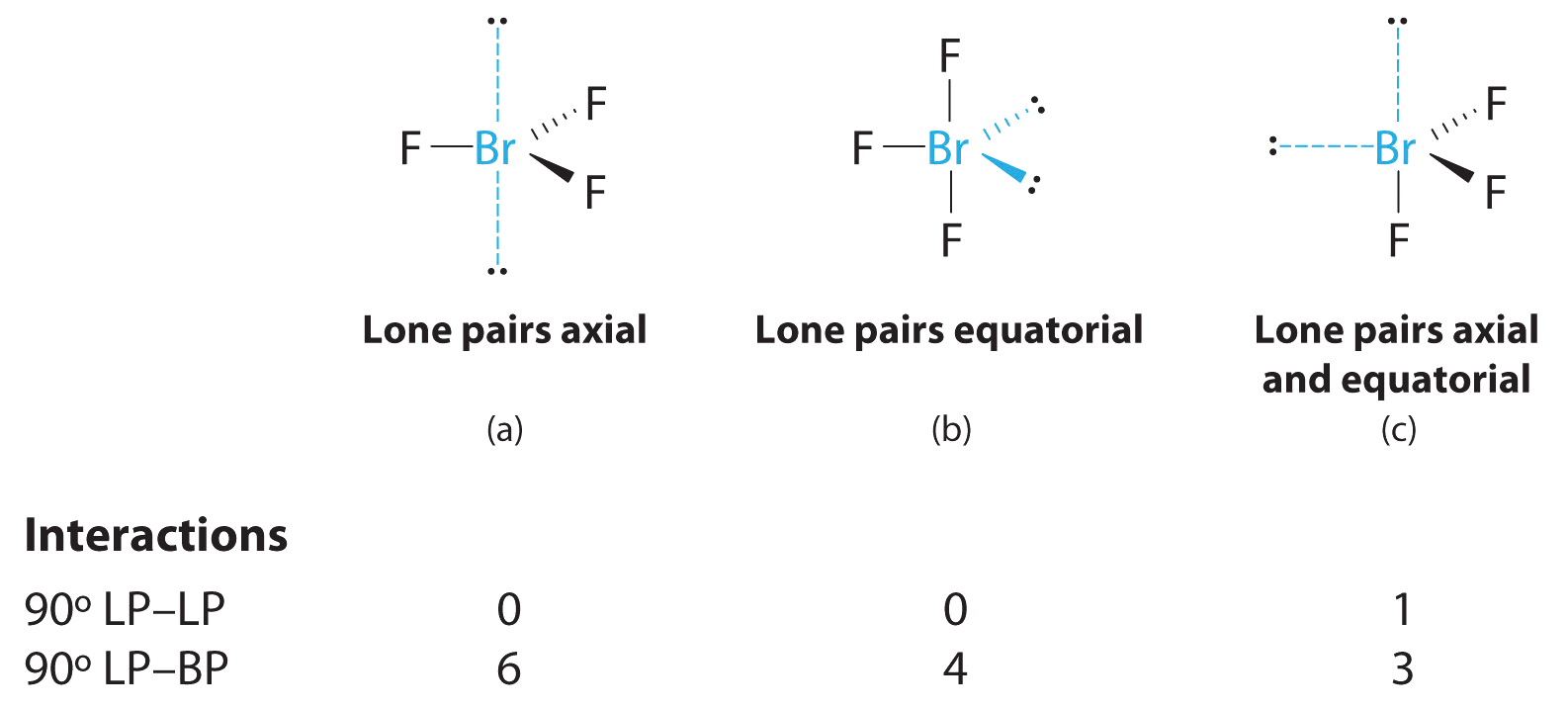
10 3 Vsper Theory The Effect Of Lone Pairs Chemistry Libretexts
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride

Brf3 Lewis Structure Bromine Trifluoride Youtube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Solution Draw The Lewis Structure Of Brf Chemistry