Ccl2o Lewis Structure
For this compound the Carbon atom in the central position and rest all the Chlorine atoms are placed around it. Calculate the total valence electrons in COCl 2 molecule.

Introduction To Covalent Bonding Lewis Theory Of Covalent
The correct answer is.

Ccl2o lewis structure. Chlorine has 7 electrons and so is 1 electron short of completely filling its outer shell. Which statement correctly describes the structure. In low concentrations its odor resembles that of freshly cut hay or grass.
Lewis dot structure 2 is the more probable most stable since there is no charge separation. The structure contains 3 single bonds 1 triple bond and 8 lone pairs. A step-by-step explanation of how to draw the Cl2O Lewis Structure Dichlorine MonoxideFor the Cl2O structure use the periodic table to find the total numb.
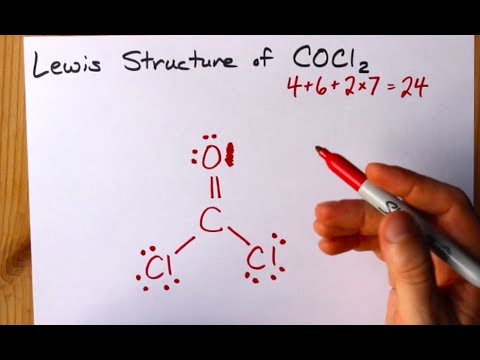
C both polar and nonpolar bonds. It is now an important industrial starting material for the synthesis of Lexan a lightweight transparent material used in bike helmets goggles and catchers masks. There are a total of 24 valence electrons for Cl 2 CO.
The O has two bonding pairs and two lone pairs and C has four bonding pairs. A step-by-step explanation of how to draw the CCl2F2 Lewis Dot Structure CCl2F2 For the CCl2F2 structure use the periodic table to find the total number o. Draw a valid Lewis structure for phosgene CCl 2 O which contains a central carbon atom.
Therefore the dot resonance structures of COCl 2 Lewis structures of COCl2 are as follows. Dichloro111Cmethanone CCl2O CID 451264 - structure chemical names physical and chemical properties classification patents literature biological activities. Phosgene is an extremely toxic gas used as a chemical weapon during World War I.
This is the structure of formaldehyde which is used in embalming fluid. The complete lewis structure of CCl2O will have. C4O6Cl2x714 Total24 Put carbon in the center.
Thus a carbon atom will share each of its 4 outer electrons with a single chlorine atom giving the single carbon atoms and 4 chlorine atoms a full outer shell of electrons. Give the Lewis structure for eqCCl_2O eq phosgene which was initially used as a chemical weapon in World War I and is now used in the plastics and pharmaceutical industries. A regular atom of carbon has 4 lone electrons in its outer shell.
In the resulting compound each element has achieved a stable electron. Draw a valid Lewis structure for phosgene CCl2O which contains a central carbon atom. The Lewis structure for Cl 2 CO requires you to place Carbon in the center of the structure since it is the most electronegative.
Write the correct Lewis dot structure for CCl2O. Rest all the non-bonding electrons are spread out in the structure. Phosgene is an extremely toxic gas used as a chemical weapon during World War I.
Write the Lewis structure for these molecules. Phosgene is a valued industrial building block especially for the production of precursors of polyurethanes and polycarbonate plastics. Both the oxygen and the carbon now have an octet of electrons so this is an acceptable Lewis electron structure.
As the central atom has four bonded pairs and sp3 hybridization the shape of the molecule is tetrahedral. The structure contains 3 single bonds 1 double bond and 2 lone pairs. Put one electron pair in each bond4.
It is now an important industrial starting material for the synthesis of Lexan a lightweight transparent material used in bike helmets goggles and catcher Click to Get Answer. B at least one double bond. The structure contains 2 single bonds 1 double bond and 2 lone pairs.
Alternatively a dot method can be used to draw the lewis structure of COCl 2. It is a colorless gas. I was wondering if COCl2 was polar or nonpolar.
Phosgene is the organic chemical compound with the formula COCl 2. Youll need a double bond between the Carbon and Oxygen atoms to acheive full outer shells for the atoms while still only using 24 valence electrons. A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure Chlorine gasFor the Cl2 structure use the periodic table to find the total number of.
A at least one lone pair on each atom. I drew its Lewis structure and got a trigonal planar shape with a double bond on the oxygen and I would normally think it was nonpolar because of the symmetrical shape however I am aware that O has a higher electronegativity than Cl so maybe the net dipole moment might be pointing towards O which would make it polar but Im not sure. The Lewis Structure Lewis Dot Diagram for CO1.
Lewis dot structure of CO Cl 2. Put least electronegative atom in centre3.

How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Ccl2o Lewis Structure How To Draw The Lewis Structure For Ccl2o Youtube

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemi Clutch Prep

Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

Representing Molecules Resonance Exceptions To The Octet Rule Formal Charge Ppt Download
Ccl2o Lewis Structure How To Draw The Lewis Structure For Ccl2o دیدئو Dideo

Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

The Complete Lewis Structure Of Ccl2o Will Have A At Least One Lone Pair On Each Atom B At Least One Double Bond C Both Polar And Nonpolar Bonds D Resonance Forms
Solved Draw A Valid Lewis Structure For Phosgene Ccl 2 O Which Contains A Central Carbon Atom Phosgene Is An Extremely Toxic Gas Used As A Chemi Course Hero
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube
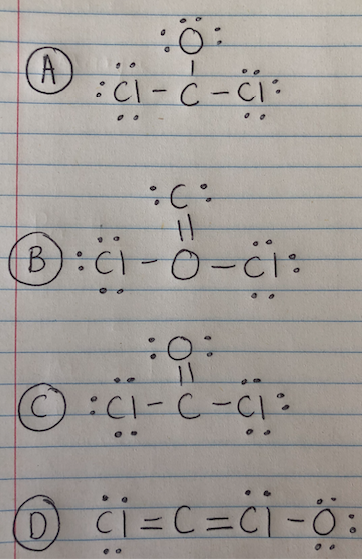
Answered Ccl2o Is A Gaseous Molecule That Is Bartleby

Cl2co Lewis Structure How To Draw The Lewis Structure For Carbonyl Dichloride Youtube

Phosgene Cl2co Is A Poisonous Gas That Was Used As A Chemical Weapon During World War I And It Is Brainly Com
Http Www Rapidlearningcenter Com Solutions Hs Chemistry Drills24 Hc Ps17 Moleculargeometry Pdf

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube
Ch2o Lewis Structure Valence Electrons Hybridization Geometry Of Molecules

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube

Draw The Lewis Structure For Ccl2o With All Resonance Forms State Its Formal Charge And Polarity What Is Its Molecular Geometry Study Com