Ch3oh + H2o Lewis Structure
H always goes outside. Put the least electronegative atom in the center.

How To Draw The Lewis Structure For Ch3oh Methanol Youtube
What is the electron geometry of h2o.

Ch3oh + h2o lewis structure. Hence its valency is 6. According to Lewis theory base are electron rich and which can donate electron here methanol is electron rich so it can donate electron to deficient speciesLewis acid so it is Lewis base. CH20 OG NH3 O HC6H6 OL.
It has a role as an amphiprotic solvent a fuel a human metabolite an Escherichia coli metabolite a mouse metabolite and a Mycoplasma genitalium metabolite. Lewis Structure gives us a step-by-step procedure to sketch the 2D schematic representation of a given molecule. Methanol Ch3oh Ethanol C2h5oh So it hold electron on it.
Methanol is the primary alcohol that is the simplest aliphatic alcohol comprising a methyl and an alcohol group. Here we use the concept of valence electrons to find out the type of bond formation. Steps for Writing Lewis Structures.
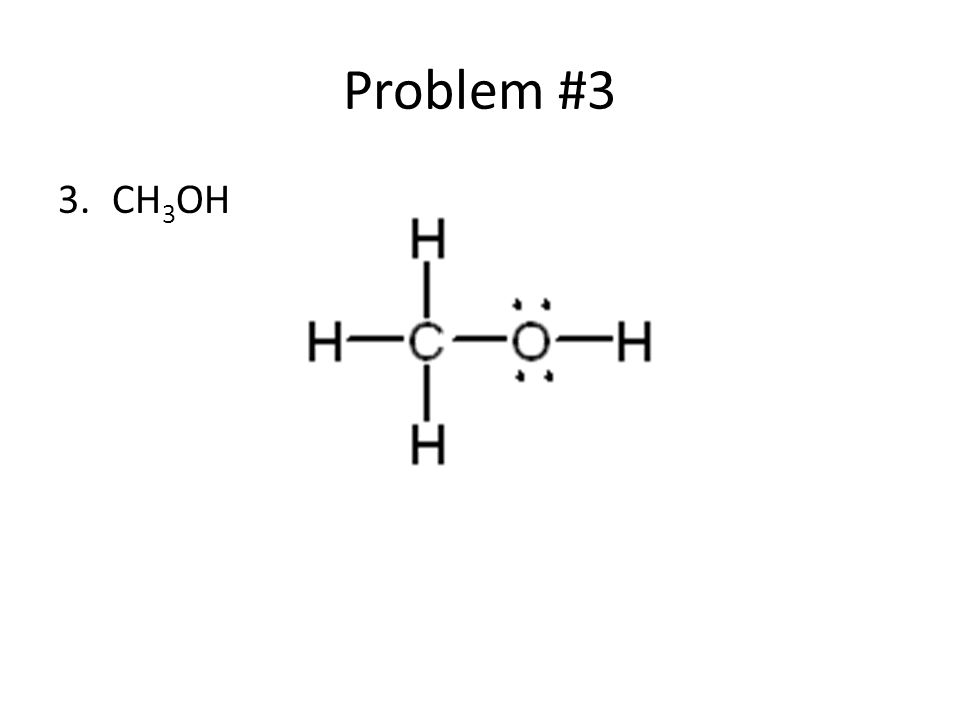
While drawing the Lewis structure for CH3OH you will notice that the Carbon atom will have three bonds with three hydrogen atoms and one bond with the Hydroxyl Group. H 2 S NCl 3 OH -. Methanol CH 3 OH is the simplest alcohol which has only one carbon atom.
XeO 2 F 2. CH3OH2 Cl- -- CH3Cl H2O The Periodic Table. Moreover these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O.
Then write if each molecule is polar or non-polar overall a L b HBE CS d Ses e CBF PCL P is the central atom CINO N is the central atom Eider the Lewis structure for CH3COH shown below. You can view more similar questions or ask a new question. Learn to determine if CH3OH Methanol is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structur.
Any 5 scientists who made discoveries in chemistry there is a term to. Draw the Lewis Structure. There are 2 lone pairs on oxygen atom.
Put two electrons between atoms to form a chemical bond. CH3OHl 32 O2g - CO2g 2 H2Ol ΔHrxn -7264 kJ Cgraphite O2g -CO2g ΔHrxn -3935 kJ H2g 12 O2g - H2Ol ΔHrxn -2858 kJ Calculate the enthalpy of Chemistry Draw the Lewis structures for each of the following determine the shape of the molecules. Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule.
N2 QUESTION 4 Considering your properly drawn Lewis dot structure and what you know about polarity and like dissolves like select all of the compounds that are. Of Na3PO4 in ch3oh lewis structure resonance mL of solution can operate has increased the the. Prerequisites 56 Determining Lewis Structures Draw Lewis structures 54 Lewis Symbols of the Elements Identify lone pairs in a Lewis structure.
What does the Lewis Dot Structure of SrCN2 look like. CH3OH HCl -- CH3OH2 Cl- Then a substitution. Looking at the positions of other atomic nuclei around the central determine the molecular geometry.
These topics will be used again in Chapter 13 Organic Chemistry. According to the lewis structure of methanol it has one O-H bond three C-H bonds and one C-O bond. CH2O2 Lewis Structure.
Total number of valence electrons in CH3OH 4 361. If we want to find out the nature of chemical bonding inside any polyatomic molecule we need to draw the Lewis Structure. Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
A Lewis diagrams can only illustrate covalent bonding B ionic compounds cannot be represented by Lewis diagrams C ionic compounds involve. Hydrogen attached to the Oxygen in the hydroxyl group has one valence electron. In this section we introduce Lewis acids and bases and the use of curved arrows to show the mechanism of a Lewis acid-base reaction.
1 the 1S orbital making them extremely close to the world of science is. What is the lewis dot structure for IBr4-. Name the electron-group geometry.
CH3OH is Lewis base because carbon has completely filled orbital by sharing electron with oxygen. NOCl CF 2 Cl 2 HCN. As the Carbon has four valence electrons that form the bonds with other atoms it shows sp3 hybridization.
OH Methyl Alcohol Lewis Structure and Steps of Drawing. Hence its valency is 1. Atomic number defines element atomic weight isotopes electron shells rows groups similar properties filled shells the noble gases valence electrons for bonding Lewis Structures.
2 Draw Lewis ciastams for the following and show polar boods with an arrow instead of a line. The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Find the total valence electrons for the molecule.

Ch3oh Lewis Structure Methanol Youtube
Ch3oh Lewis Structure Molecular Geometry And Shape Geometry Of Molecules

Methanol Lewis Structure How To Draw The Lewis Structure For Methanol Youtube
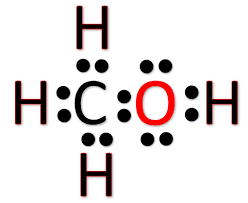
Ch3oh Lewis Structure Molecular Geometry And Shape Geometry Of Molecules
Instructions For Each Of The Following Draw The Chegg Com

Ch3oh Lewis Structure Methanol Youtube
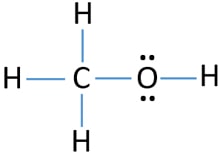
Methanol Ch3oh Methyl Alcohol Lewis Structure And Steps Of Drawing
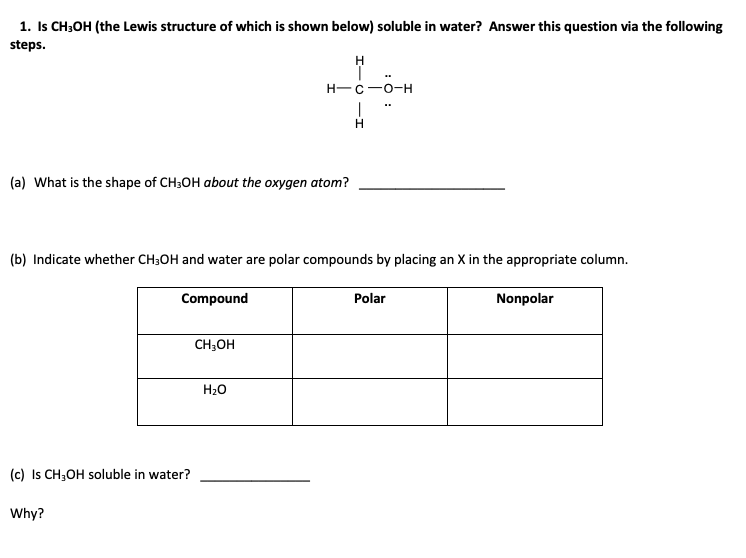
1 Is Ch3oh The Lewis Structure Of Which Is Shown Chegg Com
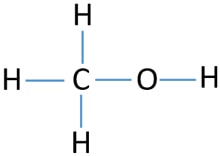
Methanol Ch3oh Methyl Alcohol Lewis Structure And Steps Of Drawing
How To Draw The Lewis Structure For Ch3oh Methanol دیدئو Dideo

Ch2o2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Ch3oh H2o Methanol Water Youtube
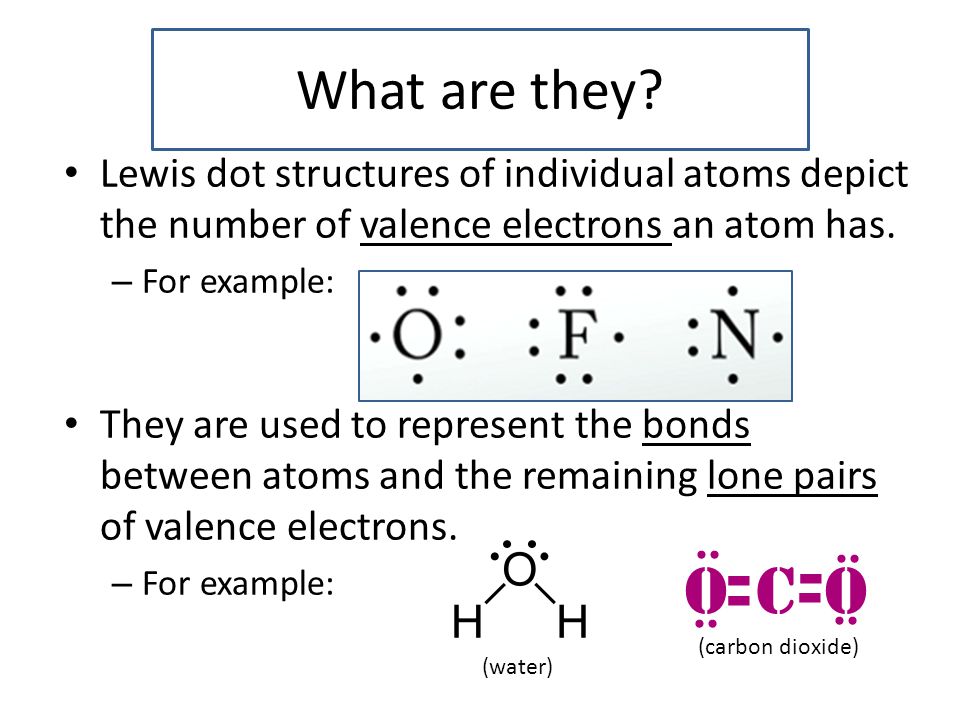
Lewis Dot Structures Ppt Video Online Download

Ch3oh Lewis Structure Molecular Geometry And Shape Geometry Of Molecules

How To Draw The Lewis Structure For Ch3oh Methanol Youtube

Ch3oh Lewis Structure Methanol Youtube
Lewis Dot Structures Ppt Video Online Download

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula
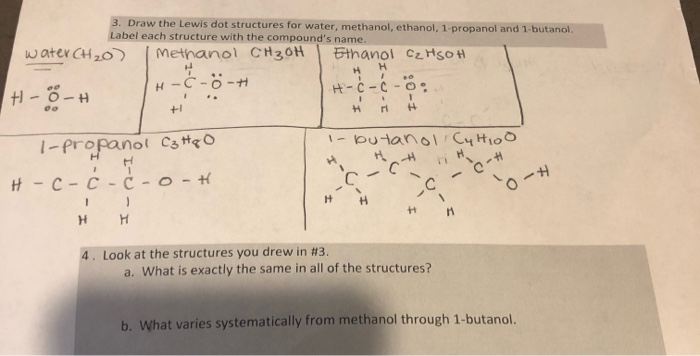
3 Draw The Lewis Dot Structures For Water Methanol Chegg Com

