Clf5 Lewis Structure Polar Or Nonpolar
Which one is it REALLY and WHY. 1 Answer to BrCl5 bromine pentachloride is sometimes called a polar molecule sometimes called nonpolar.
Clf5 polar or nonpolar.

Clf5 lewis structure polar or nonpolar. Formal charge Valence electrons unbonded electrons 12 bonded electrons Now we will calculate the formal charge on carbon which is the central atom in the CF4 lewis structure. Quiz your students on Lewis Dot Diagram Structure For ClF5 Molecular Geometry Bond Angle Hybridization PolarNonpolar using our fun classroom quiz game Quizalize and. Draw the 3D molecular structure w VSEPR rules Step 3.
The total valence electron is available for drawing the Aluminium chloride AlCl3 lewis structure is 24. It is polar because ClF5 has square pyramidal shape as steric number is 6 and it has 1 lone pair. Clf5 polar or nonpolar.
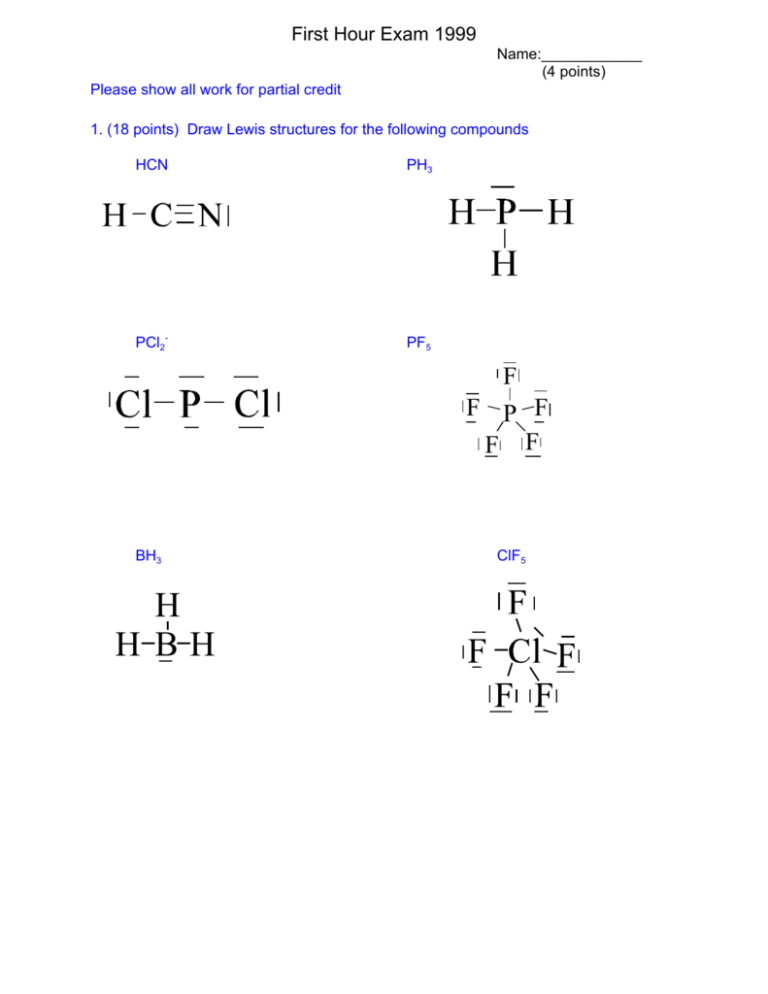
Draw the Lewis structure for ClF5 in the. Chlorite has a 3 oxidation state and is part of the chlorine oxides family. For Chlorine we look on the periodic table and it has 7 valence electrons.
February 28 2021. Home Uncategorized clf5 polar or nonpolar. Single Blog Title This is a single blog caption.
Chlorite is used in the paper pulp and bleaching of textiles. A- Draw the Lewis structure for SOF4 in the window below and then decide if the molecule is polar or nonpolar. A Cl-F bond is a dipole since F is more electronegative.
Properties of Chlorite ion. But it doesnt matter start placing the remaining electron around any chlorine atom as you prefer. Use symmetry to determine if the molecule is polar or non-polar.
Answer CLF5 CHLORINE PENTAFLUORIDE is Polar What is polar and non-polar. As Cl2 lewiss structure only contain two atoms that are similar so you can assume any of one is central and the other one is the outer atom. In this article we will study Chlorite ionClO2- lewis structure molecular geometry hybridization polar or nonpolar bond angle etc.
A polar molecule with two or more polar. Question Is CLF5 polar or nonpolar. It is used in various rockets and chemicals.
By Staff Writer Last Updated Mar 31 2020 33755 PM ET. Is BrF5 Polar or Nonpolar. BrF5 is very toxic to inhale and severely corrosive to the skin.
Draw the Lewis structure Step 2. 9- MOLECULAR POLARITY. The molecule is polar and has polar bonds.
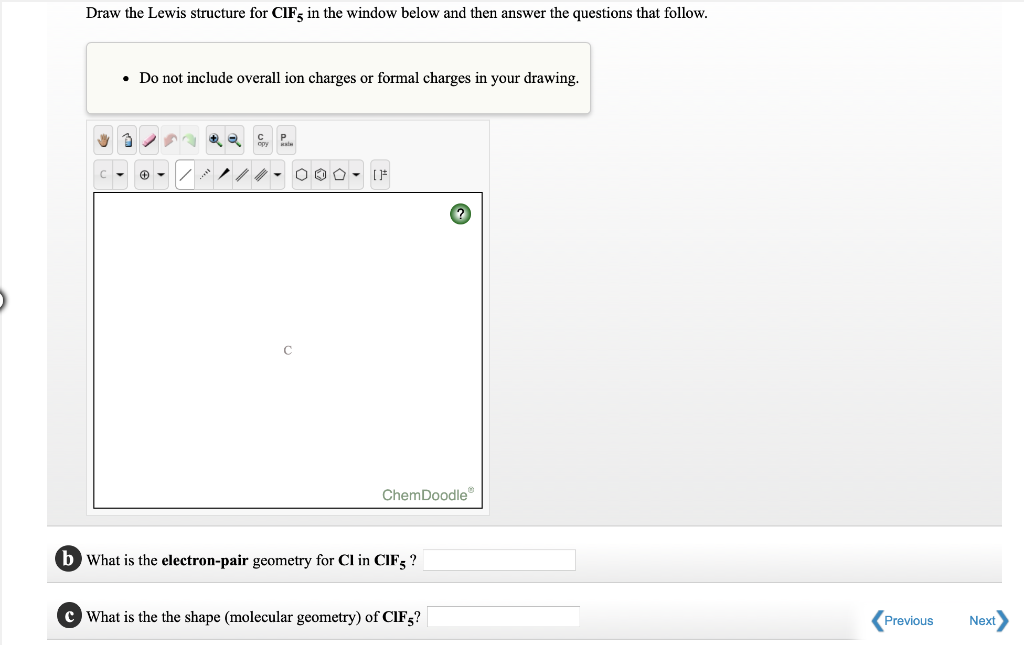
Draw the Lewis structure for ClF5 in the window below and then answer the questions that follow. Use this equation given below. For the ClF5 Lewis structure there are a total of 42 valence electrons available.
Click on the molecules name to see the answer but first try to do it yourself. BrF5 is a polar molecule because negative charge is not distributed equally around the molecule. Long-term exposure to this can cause liver failure or kidney damage.
The hybridization of the AlCl3 molecule is Sp 2 since it has a steric number equal to 3 that will form an Sp 2 hybrid. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. It has a molar mass of 67452 g.
In this article we will study Bromine pentafluoride BrF5 lewis structure molecular geometry polar or non-polar its hybridization etc. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Ill tell you the polar or nonpolar list below.
So on this behalf BrF5 is Polar without a doubt. Is CLF5 polar or nonpolar. C The molecule is polar and has nonpolar bonds.
But each chlorine already sharing two electrons with the help of a. To identify and have a complete description of the three-dimensional shape of a molecule we need to know also learn about. Here is a list of molecules that are considered.
Correct answer to the question Identify all intermolecular forces that exist. In phenol OH group is present on benzene ring. In chemistry polarity is a separation of.
To calculate the formal charge in CF4 lewis dot structure. The central atom is sp² hybridized with one lone pair. We have 12 remaining valence electrons and each chlorine needs 8 electrons for completing its octet.
In square pyramidal shape the 4 fluorine atoms in a plane forming a square give net dipole zero but one fluorine atom and lone pair give rise to net dipole moment of the molecule. AlCl3 is a nonpolar molecule because its net dipole moment is zero and charges are uniformly distributed all over the atom. SF 5 Cl - Sulfur Monochloride Pentafluoride.
B- Draw the Lewis structure for BrF3 in the window below and then answer the questions that follow. By Depending on the arrangement of outer atoms this molecule could be polar or nonpolar.

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry
Draw The Lewis Structure For Clf5 In The Window Below Chegg Com
Is Clf5 Polar Or Non Polar Quora
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Lewis Dot Structure General Chemistry Quiz Docsity

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation
Is Clf5 Polar Or Non Polar Quora

Clf5 Lewis Structure How To Draw The Lewis Structure For Clf5 Chlorine Pentafluoride Youtube
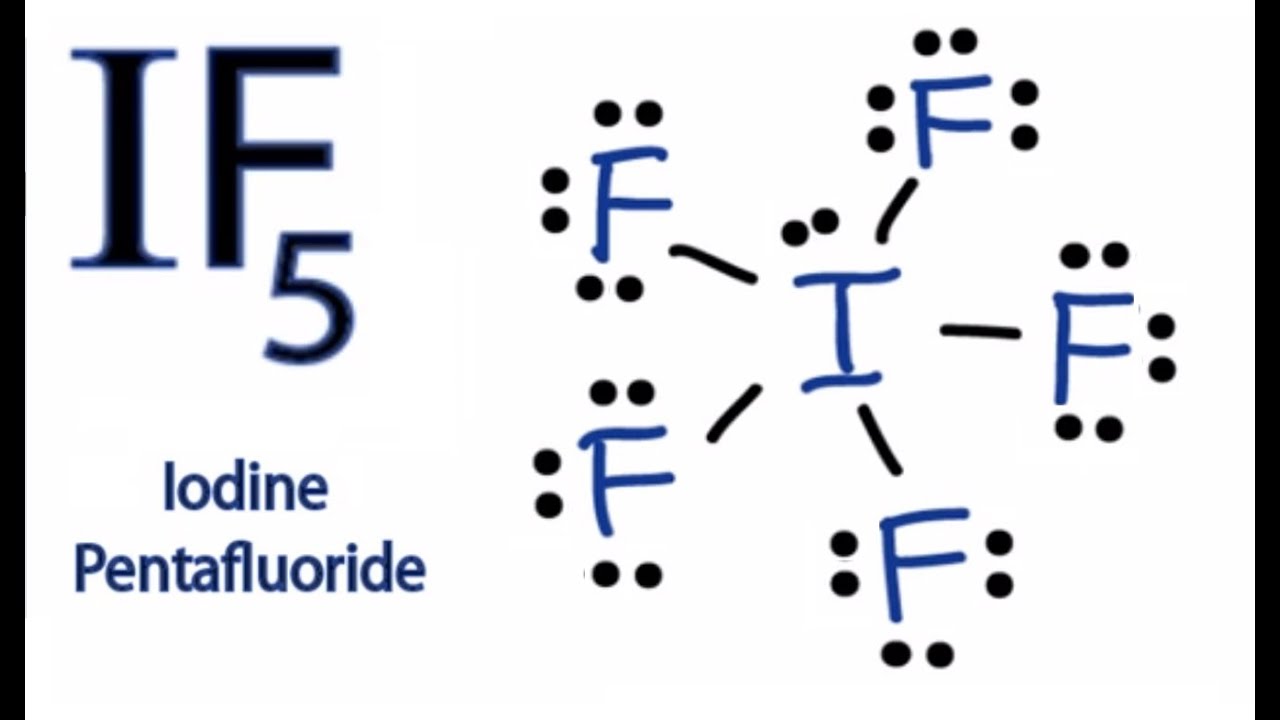
If5 Lewis Structure How To Draw The Lewis Structure For If5 Youtube

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
Http Chemvision Net 50 Hmw Solutions Ch7 Pdf

Cf4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Chlorine Pentafluoride Clf5 Lewis Structure Lewis Structure For Brf5 Molecular Geometry Bond Angle Hybridization Polar Or Nonpolar Just Another