Does C2h2 Have Resonance Structures
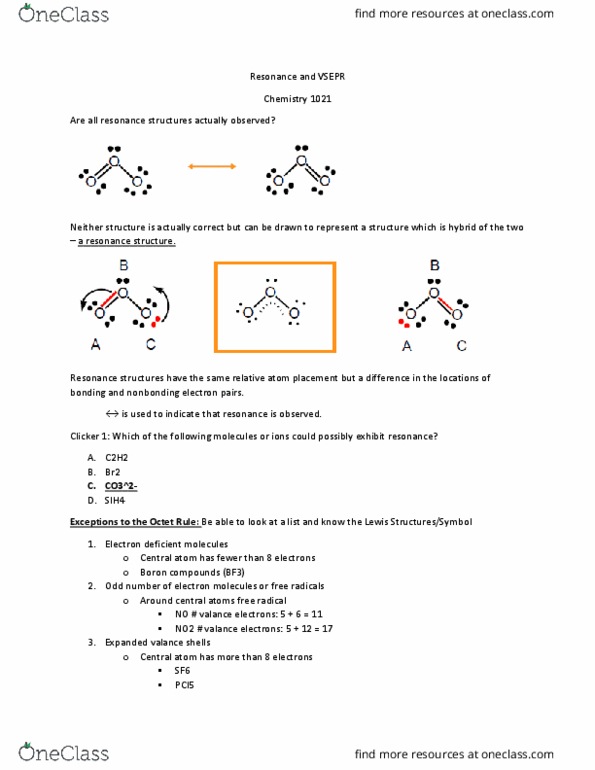
When we draw resonance structures we convert lone pairs to bonds and bonds to lone pairs if it is possible. There is no resonance structure of CO2 as carbon forms double covalent bond with both oxygen atoms at its two opposite sides it forms linear structure.
Draw The Lewis Structure For C2h2 Cio 3 Sf4 Chegg Com
A molecule has resonance if more than one lewis structure can be drawn for that molecule.

Does c2h2 have resonance structures. Is C2H2 a resonance. Since there is only one possible lewis structure C2H2 does not have resonance. In lewis structure of N 2 O 4 ion there are two double bonds NO between oxygen and nitrogen two N-O single bonds and one N-N bond.
N a B r. Lone pairs charges and bonds of N 2 O 4 molecule. Since there is only one possible lewis structure C2H2 does not have resonance.
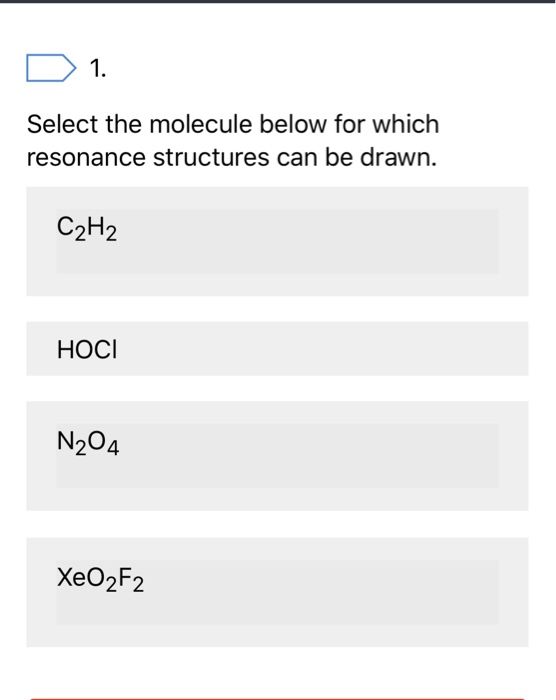
Select the molecule below for which resonance structures can be drawn. For molecules with resonance each lewis structure individually does not accurately depict the structure. C X 6 H X 12.
Which compound has resonance structures. C2H2 HOCI N204 XeO2F2. Drawing the Lewis Structure for C 2 H 4.
The other choices are equally confusing. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. The Lewis model does not account for some behavior of molecules such as O2 being paramagnetic.
A molecule has resonance if more than one lewis structure can be drawn for that molecule. See the answer See the answer See the answer done loading. In two oxygen atoms each have three lone pairs with a -1 charge.
VSEPR theory accounts for the shapes of molecules but does not tell us how those shapes come about. It has resonance structures but no isomers. Since there is only one possible lewis structure C2H2 does not have resonance.
N a X 2 C O X 3. It doesnt have any other structure so resonance structure is impossible to form. The answer key says D is correct but I do not understand why N a X 2 C O X 3 which is an ionic compound between the polyatomic ion carbonate and sodium can have resonance structures.
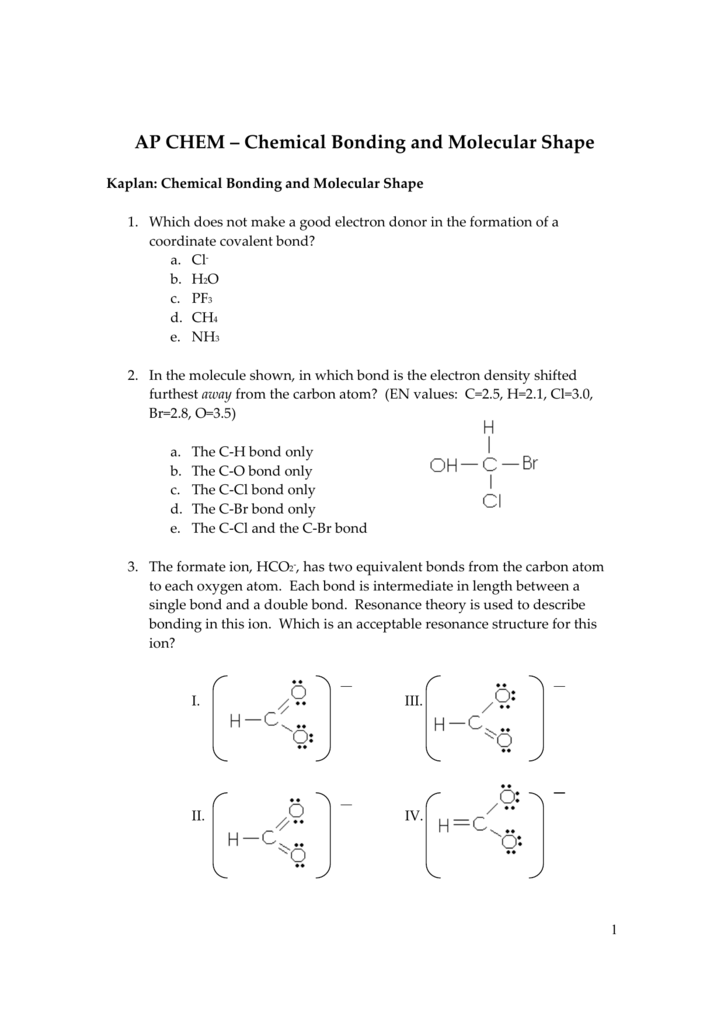
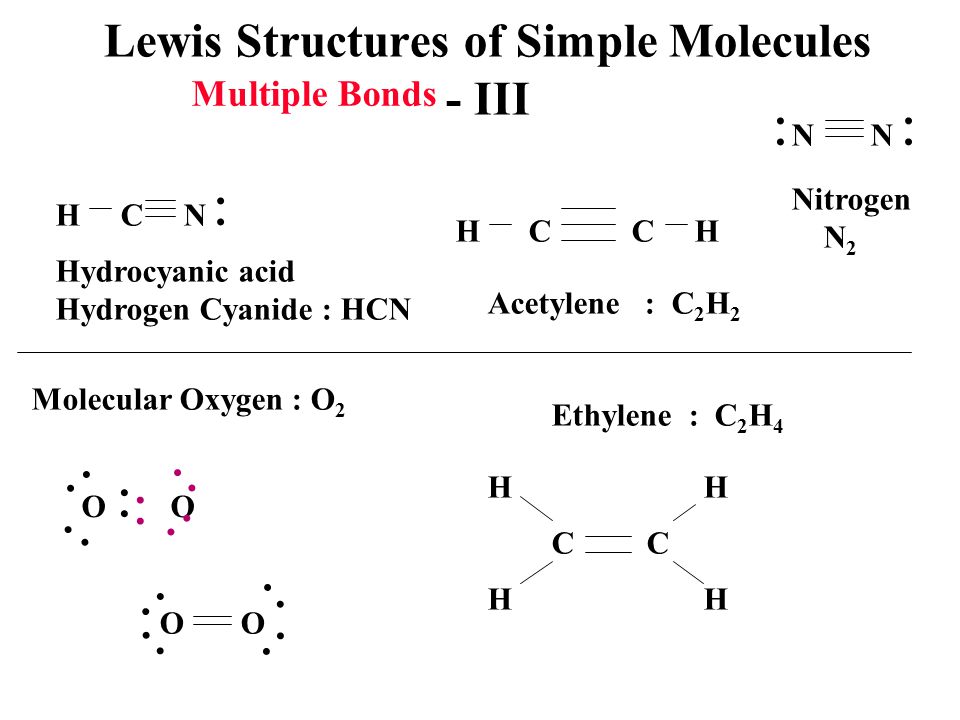
C H X 3 C H O. A dual temperature and lightresponsive C 2 H 2 C 2 H 4 separation switch in a diarylethene metalorganic framework MOF is presented. For C 2 H 4 you have a total of 12 total valence electrons.
This problem has been solved. For molecules with resonance each lewis structure individually does not accurately depict the structure of the molecule. A molecule has resonance if more than one lewis structure can be drawn for that molecule.
These resonance structures differ only in the position of electrons but not in the position of nuclei. For molecules with resonance each lewis structure individually does not accurately depict the structure. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Select the molecule below for which resonance structures can be drawn. The simple Lewis structure VSEPR model of bonding is easy to picture but it has limitations. C2H2 HOCI N204 XeO2F2.

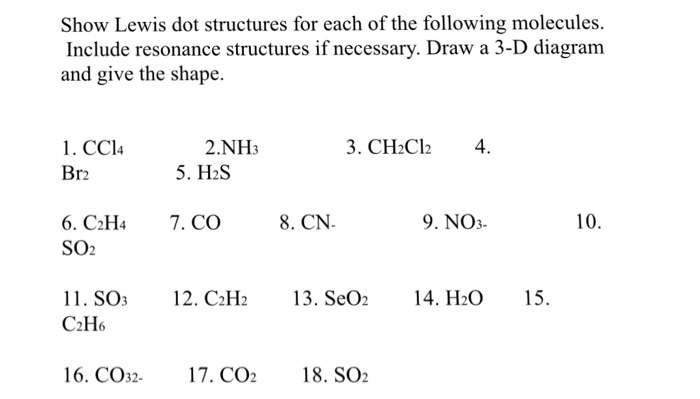
Isomers Or Resonance Structures Lewis Structure Chegg Com

Lewis Structure For C2h2 Ethyne

C2h2 Lewis Dot Structure Geometry Youtube
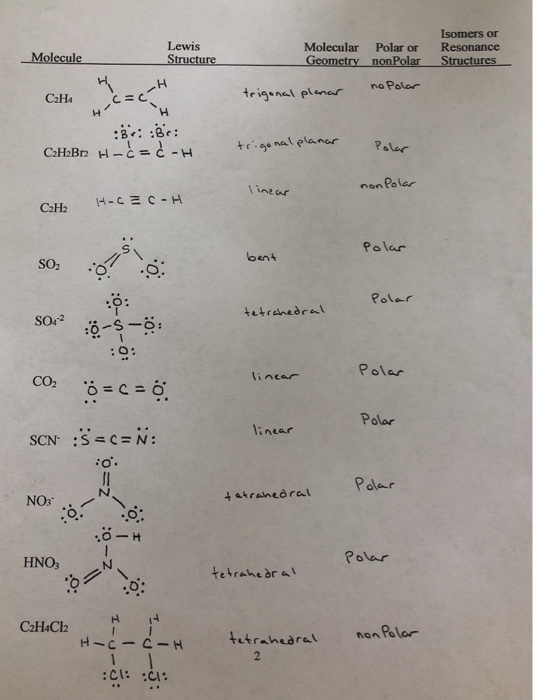
I Got Most Of These Problems But Im Stuck On Finding Chegg Com

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

C2h2 Acetylene Ethyne Lewis Structure
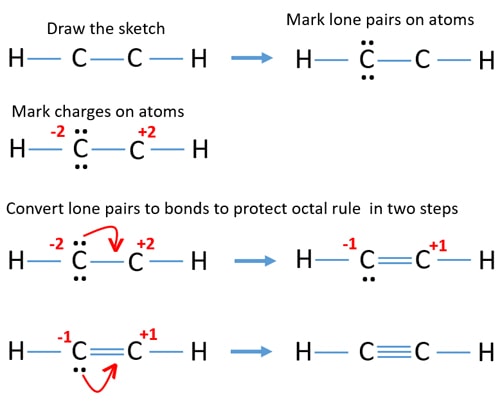
C2h2 Acetylene Ethyne Lewis Structure

How To Calculate The Formal Charges For C2h2 Ethyne Youtube
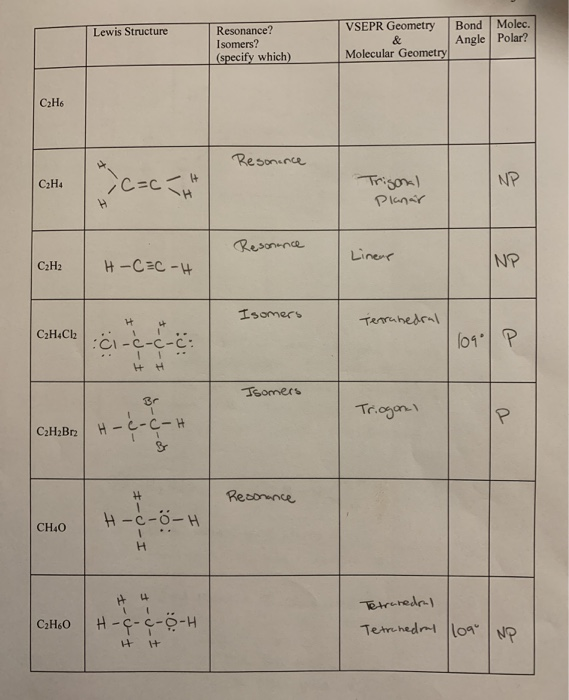
Lewis Structure Bond Molec Angle Polar Resonance Chegg Com
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Draw The Orbital Overlap Diagram Of C2h2 Ethyne Acetylene Youtube
1 Select The Molecule Below For Which Resonance Chegg Com
Show Lewis Dot Structures For Each Of The Following Chegg Com
Lewis Structure For C2h2 Ethyne