Does Pcl5 Follow The Octet Rule
Does PCl5 follow the octet rule. So we need 2 more covalent bonds to form an octet.

Does Pcl5 Follow The Octet Rule
Why is PCl5 an exception to the octet rule.
Does pcl5 follow the octet rule. Does pcl3 violate the octet rule. Co has only 6 electrons while PCl5 has 10 electrons after sharing so both dont follow octet rule. The octet rule is based upon available n s and n p orbitals for valence electrons 2 electrons in the s orbitals and 6 in the p orbitals.
The main idea behind the octet rule was that Noble gases are inert hence stable and other atoms tend to attain stable electronic configurationBut even noble gases like Xenon and Radon form compounds. Which of the following does not obey octet rule. An octet corresponds to an electron configuration ending with s2p6.
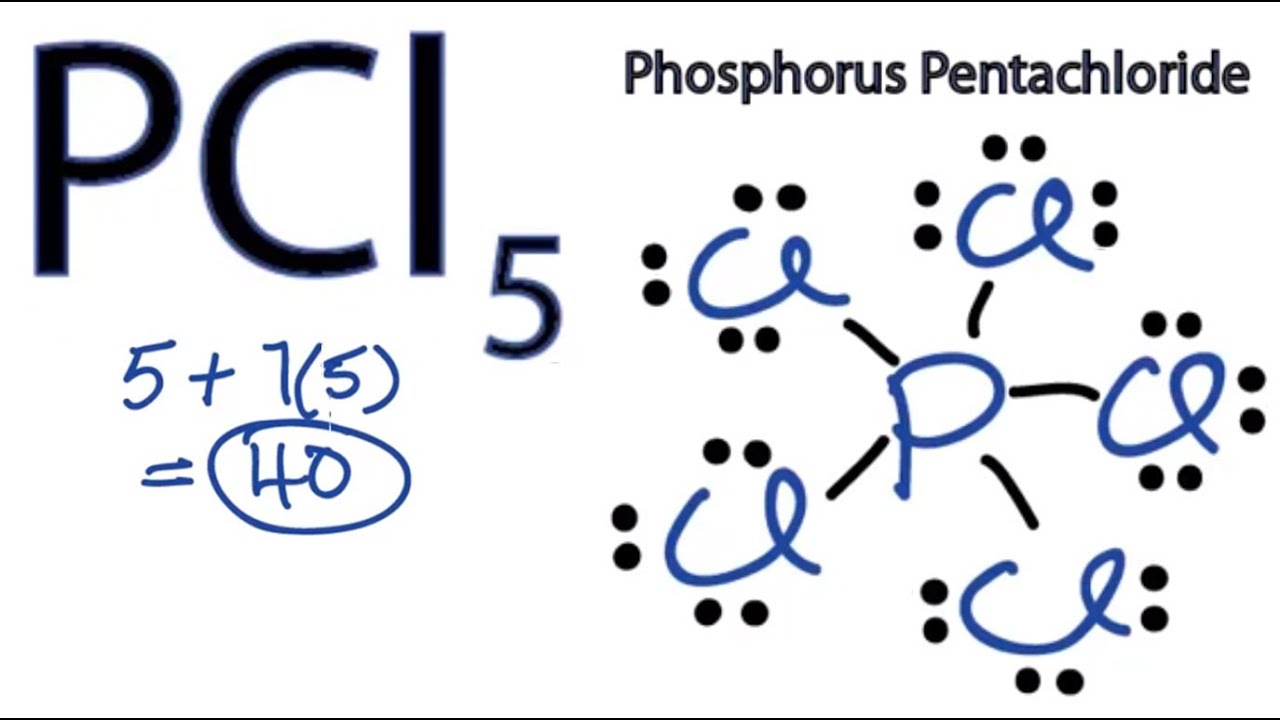
1 question Indicate which of the following substances contain an atom that does or does not follow the octet rule. For example P C l 5 is a legitimate compound whereas N C l 5 is not. When drawing the Lewis structure for PCl5 five chlorine Cl atoms are bonded to the central atom phosphorous P.
In the PCl 5 molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule. Oxygen has 6 valence electrons the bonds should be 8-62 bonds. Expanded valence shells are observed only for elements in period 3 ie.
Does oxygen follow the octet rule. PCl_5 The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. Is PCl5 a Lewis acid.
So O2 does not satisfy the octet rule because as we know octet rule states that an atom has to have 8 e- in the outer shell. Whereas in PCl5 there will be two electrons left over which is why it is an exception to the octet rule. Those atoms can be the same element as when oxygen bonds with itself to form O2 or with different elements such as water H2O.
Does H2O follow the octet rule. Out of all the molecules given only BrF 4 4 does not follow octet rule. Which atoms can exceed the octet rule.
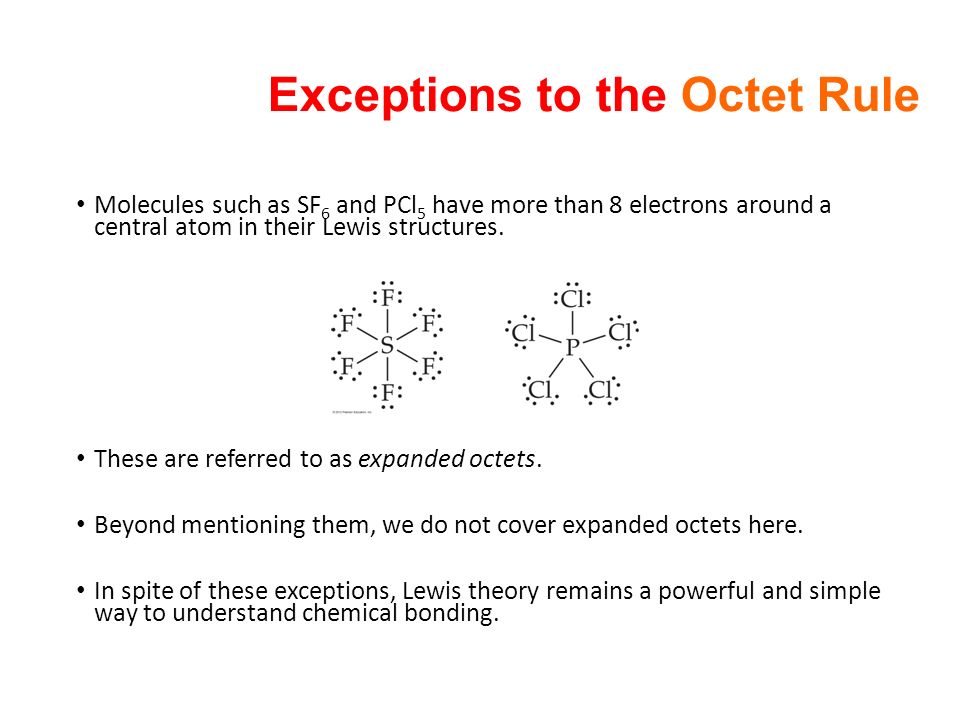
So only the octet of oxygen. Phosphorus pentachloride PCl5 and sulfur hexafluoride SF6 are examples of molecules that deviate from the octet rule by having more than 8 electrons around the central atom. BCl3 forms Lewis adducts.
Because of an empty p-orbitalB accepts electronsThus is a strong Lewis acid. The Octet Rule is a general rule that is used to describe chemical bonding and draw Lewis Structures. Sulfur phosphorus silicon and chlorine are common examples of elements that form an expanded octet.
Does pcl5 follow octet rule. The chlorine atoms obey the octet rule but the phosphorus atom does not. The rule states that Main Group elements form bonds in.
The structure of the compound is -. Of electrons underlinecolorwhitebbaround central atom colorwhiteCH_4 colorwhite 4 2 8 colorwhitePCl_5 colorwhite 5 2 colorblue10 colorwhiteNH_3. As a result the second period elements more specifically the nonmetals C N O F obey the octet rule without exceptions.
How many electrons does an atom need to accomplish the octet rule. Since Cl has seven valence electrons it can take on one more to accomplish an octet. In the PCl5 molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule.
I would like to know why P can exceed the number of valence electrons. Also the octet rule is an upper limit for atoms without d-orbitals. Phosphorus pentachloride PCl5 as exception to octet rule.
Phosphorus pentachloride PCl5 and sulfur hexafluoride SF6 are examples of molecules that. In PCl3 Ps three valence electrons are evenly distributed among the three chlorine atoms. Does pcl3 violate the octet rule.
In the PCl5 molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule. Therefore does not follow octet rule and hence is an exception to this rule. In a molecule of phosphorus pentachloride P Cl5 each chlorine atom ends up with an octet of electrons four pairs but the phosphorus atom ends up with ten electrons five pairs.
Postby Julie Nguyen 1B Mon Jul 11 2016 728 am. Because it is on the period 3 so it order to have the lowest energy structure the pcl5 can break the octet rule.

Exceptions To The Octet Rule Boundless Chemistry

Chemistry Octet Rule Homework Help Science Forums
Https Www Topperlearning Com Answer How Is Pcl5 A Covalent Bond Why Is The Valence Electrons In Phosphorus Making 10 Instead Of 8 Why Doesnt It Follow The Octet Rule Why Is It Non Polar K7uhcqzz
Does Pcl3 Satisfy The Octet Rule Quora
How Is The Octet Rule Used Quora
Structures For H2co3 Sf6 Pf5 And If7 Is The Octet Rule Obeyed In All These Cases Sarthaks Econnect Largest Online Education Community
Does Pcl5 Follow The Octet Rule Quora
Does Pcl5 Follow The Octet Rule Quora
Http Pubs Rsc Org En Content Articlepdf 2015 Sc C5sc02076j Page Search

Pcl5 Does Not Obey Octet Rule But It Is Stable Plz Explain How And Why Chemistry Chemical Bonding And Molecular Structure 11581006 Meritnation Com
Does Pcl3 Satisfy The Octet Rule Quora
Chapter 8 Basic Concepts Of Chemical Bonding Ppt Download
How Is The Octet Rule Used Quora

Introductory Chemistry Fourth Edition Nivaldo J Ppt Download
Does Pcl5 Follow The Octet Rule Quora
Does Pcl5 Follow The Octet Rule Quora

Does Pcl5 Follow The Octet Rule