Draw The Structure Of H2o2 Molecule
Thus in the liquid state H 2 O exists as an associated liquid. Bent first draw the lewis structure of water.
Is H2o2 Polar Or Non Polar Quora
The molecule is also not built in a planar manner but rather twisted.

Draw the structure of h2o2 molecule. A step-by-step explanation of how to draw the O2 Lewis Dot Structure Oxygen Gas Diatomic OxygenFor the O2 structure use the periodic table to find the t. Draw it and label the varous bond angles and bond length. In the first step we need to calculate how many valence electrons present in.
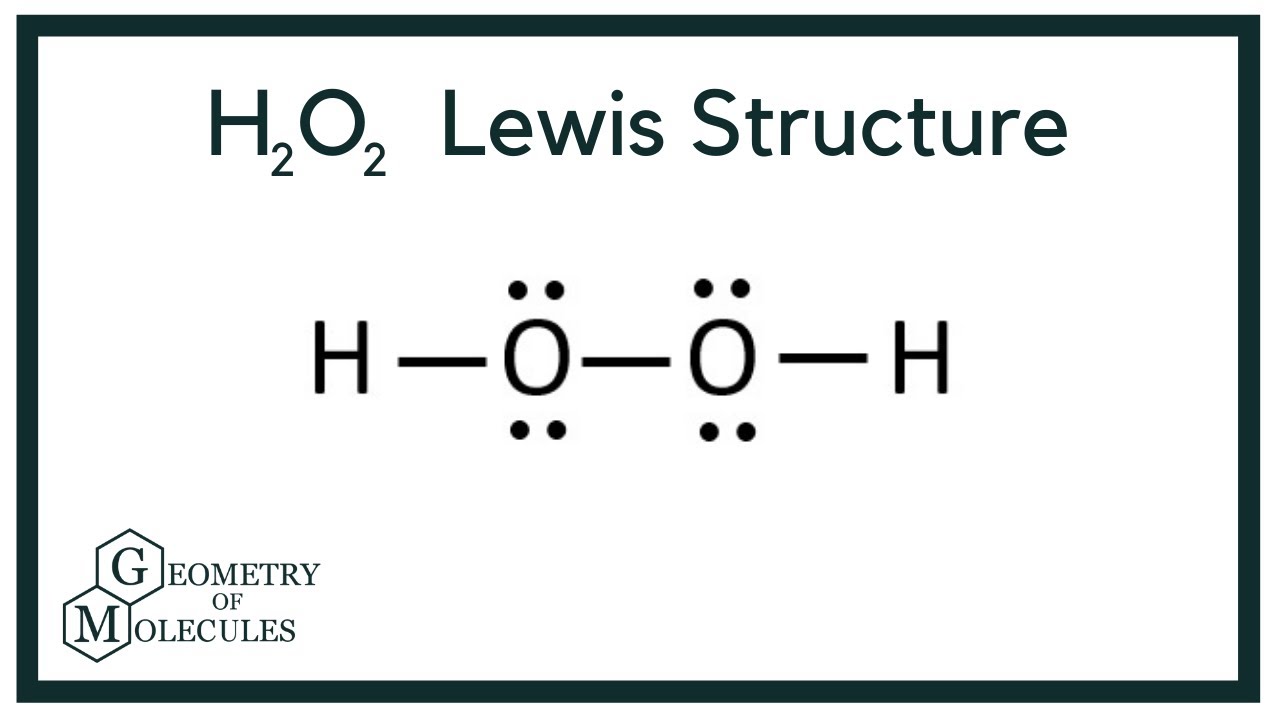
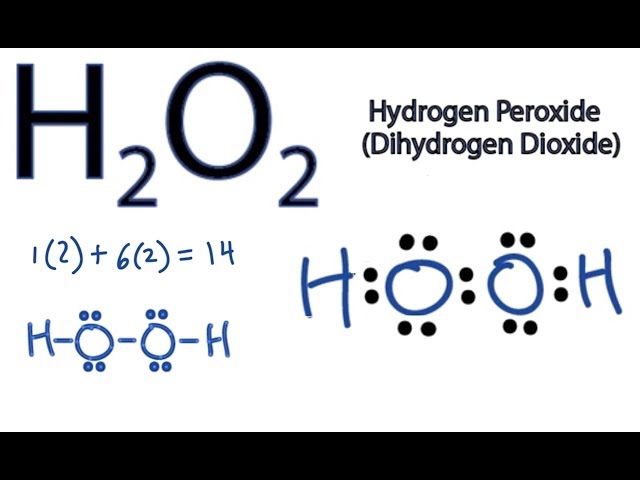
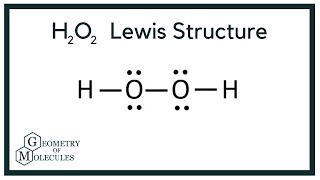
H2O2lewis structure There are two hydrogen atoms and two oxygen atoms in H2O2. Each step of drawing lewis structure of H2O2is explained in detail in this tutorial. A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure Hydrogen peroxide.
The OH bond length is 957 pm. A step-by-step explanation of how to draw the H2 Lewis Dot Structure Hydrogen gasFor the H2 structure use the periodic table to find the total number of v. To draw the lewis structure of this molecule valence electrons of hydrogen and oxygen are counted.
The structure must have a total of 8 valence electrons because there are 2. H2o lewis structure dot molecular diagram water electron geometry draw bond vsepr valence chemical example molecule charge chemistry electrons theory h2o water 2d structure svg molecules chemical lewis molecule diagram chemistry bonds covalent os monoxide dhmo pixels molecular shape wikimedia. Water molecule has bent geometry with H-O-H bond angle of 1 0 4.
In the liquid state H 2 O molecules are held together by intermolecular hydrogen bonds. Find the least electronegative atom and placed it at center. In other words each water molecule is generally H-bonded to four other water molecules.
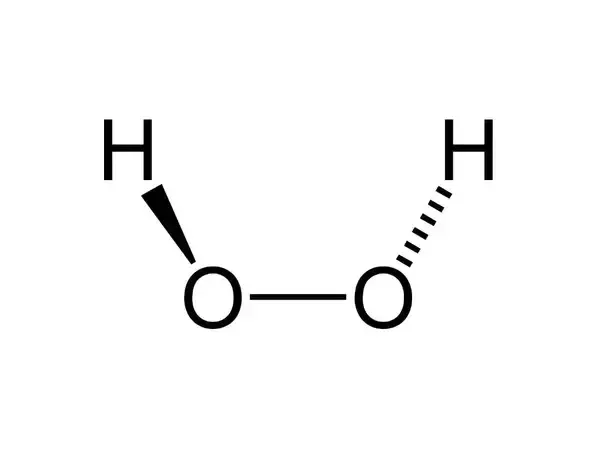
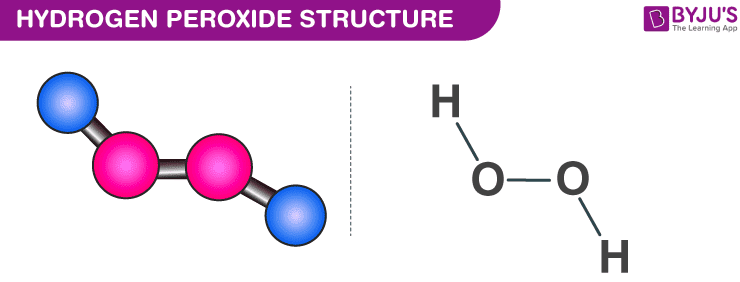
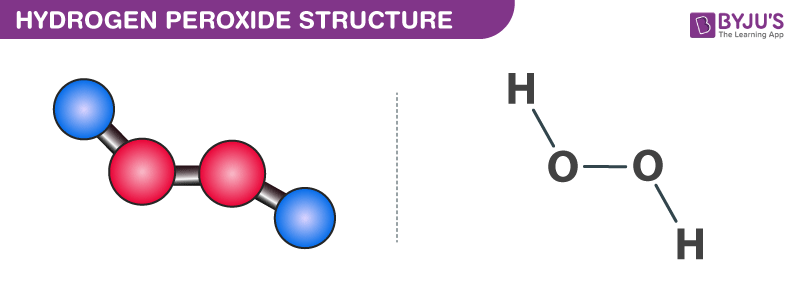
The structure of hydrogen peroxide is non-planar. It has non-planar structure. Follow some steps to drawing the H2O2 Lewis dot structure 1.
Hydrogen peroxide has a non-planar structure both in gas and solid phase. In the crystal the dihedral angle 1115 reduces to 902 on account of hydrogen bonding. H 2 O 2 has an open book structure with O O spins.
Hydrogen atoms are joint to oxygen atom through single bonds. Apr 17 2014 - A step-by-step explanation of how to write the Lewis Dot Structure for H2O2 Peroxide or Dihydrogen Dioxide. A single covalent bond is present between 2 O atoms.
Draw it and label the varous bond angles and bond length. Drawing the lewis structure for h 2 o. The bond angle H-O-O in the molecule of H2O2 is only 948.
H2os lewis dot structure gives it many. It is helpful if you. In gaseous phase water molecule H2O has a bent form with a bond angle of 1045.
What type of structure is possessed by H_2O_2 molecule. The dihedral angle in gas and solid phase is 1115 and 902 respectively. Only in this way is the mutual repulsion of the H atoms and the big non-bonding sp 3 orbitals the least.
Each O atom is covalently bonded to one H atom. The given molecule is named hydrogen peroxide. The structure of H 2 O 2 is non planar.
In order to determine the molecular geometry for h2o observe the lewis structure of the same. Draw a Lewis dot electron dot structure for a molecule of hydrogen peroxide H2O2. The dihedral angle is 111.
There is single bond between two oxygen atom in hydrogen peroxide. In the crystal the dihedral angle 1115 reduces to 902 on account of hydrogen bonding. In the lewis structure of H 2 O there are two single bonds around oxygen atom.
Whenever hydrogen present in any molecule then it. There is an atom of oxygen in the center and two atoms of hydrogen around the central atom. Note that the H2O2 Lewis structure is frequently used on tests a.
A hydrogen atom is made a bond with one oxygen atom. For the H2O2 Lewis structure calculate the tot. Two O-H bonds are in different planes and the dihedral.
A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc. The structure of H2O can be shown as. H-O-O-H where - represents the bond So there is polarity between the H and O atom but since H2O2 is not symmetrical there is some net polarity.
Comment on the dipole moment of H_2 O_2 molecule. Structure of H2O2 is. Count total valence electron in H2O2.
This angle around the O-O axis is 1115. Also there are two lone pairs on. Each oxygen atom can form two H-bonds using each of its two lone pairs of electrons.
The O-O bond length is 1458 pm and the O-H bond length is 988 pm which is equal to 988 10 -13 m.
H2o2 Lewis Structure Hydrogen Peroxide Youtube

Is H2o2 Polar Or Non Polar Quora

Hydrogen Peroxide Structure Properties Uses With Questions Videos
H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Youtube
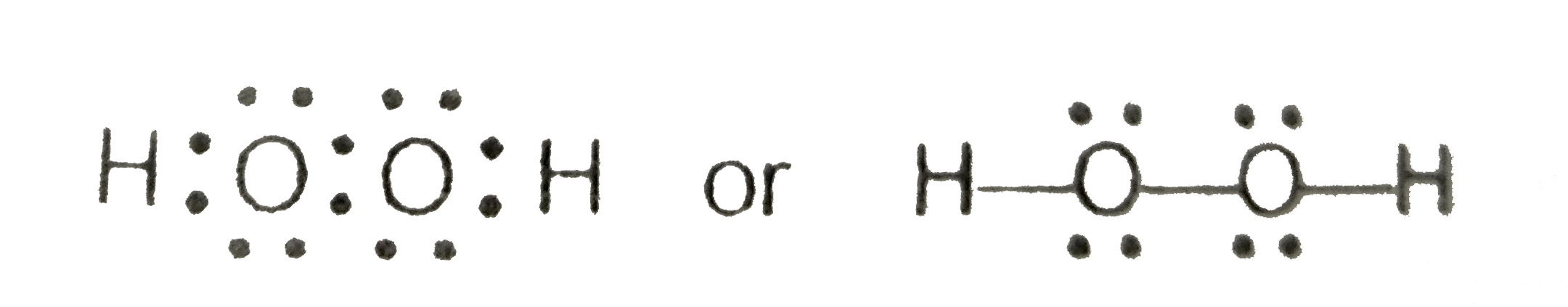
Write The Lewis Structure Of Hydrogen Peroxide

Figure 1 From Aquaporin Facilitated Transmembrane Diffusion Of Hydrogen Peroxide Semantic Scholar
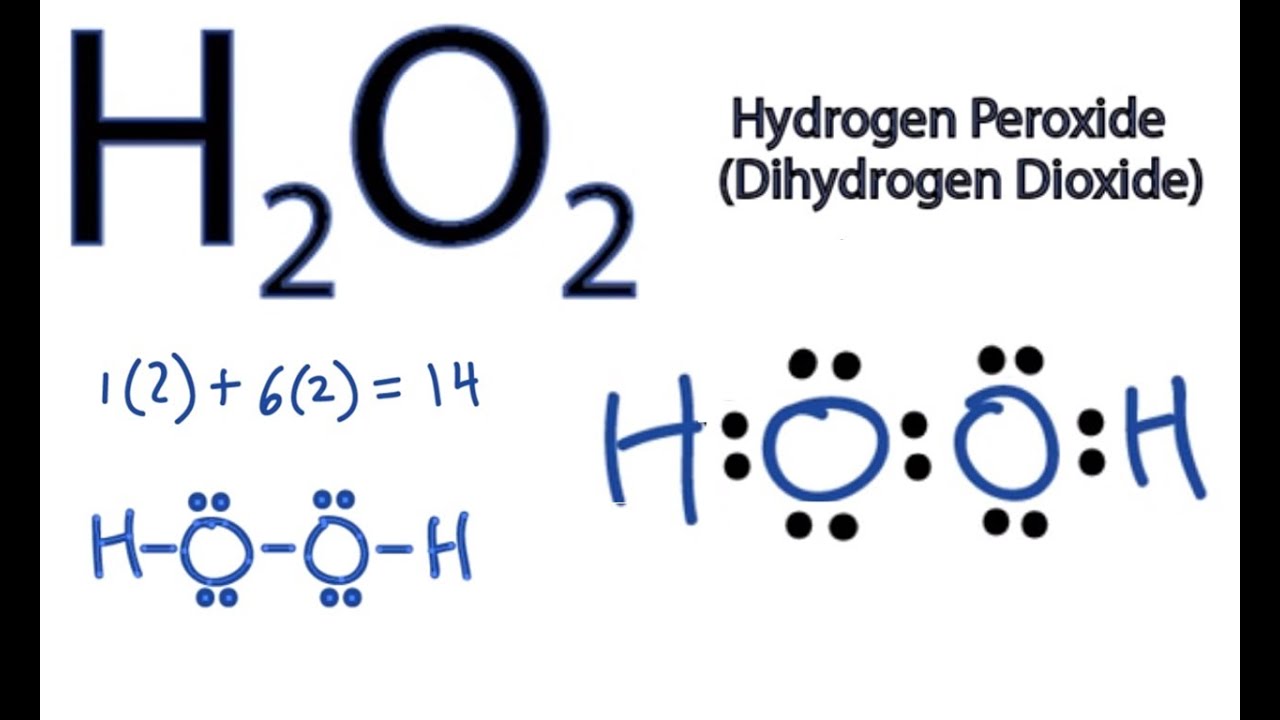
Diagram Dot Diagram Of H2o2 Full Version Hd Quality Of H2o2 Diagramrt Bmwe21fansclub It
Hydrogen Peroxide Molecule Of The Month September 2006 Html Version

Stock Illustration Hydrogen Peroxide H2o2 Molecule Clipart Drawing Gg76414335 Gograph
Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
Chemical Makeup Of Hydrogen Peroxide Saubhaya Makeup
H2o2 Lewis Structure Hydrogen Peroxide Youtube
H2o2 Lewis Structure Hybridization Molecular Geometry And Bond Angle

H2o2 Shape And Polarity Https Ift Tt 3c7p6s4 Molecular Geometry Molecular Shapes Intermolecular Force

Draw The Lewis Structure For Hydrogen Pero Clutch Prep

Hydrogen Peroxide Structure H2o2 Over 100 Million Chemical Compounds Mol Instincts

What Is The Structure Of H2o2 Draw A Schematic Diagram Indicating The Shape Of The Molecule Clearly

H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity
